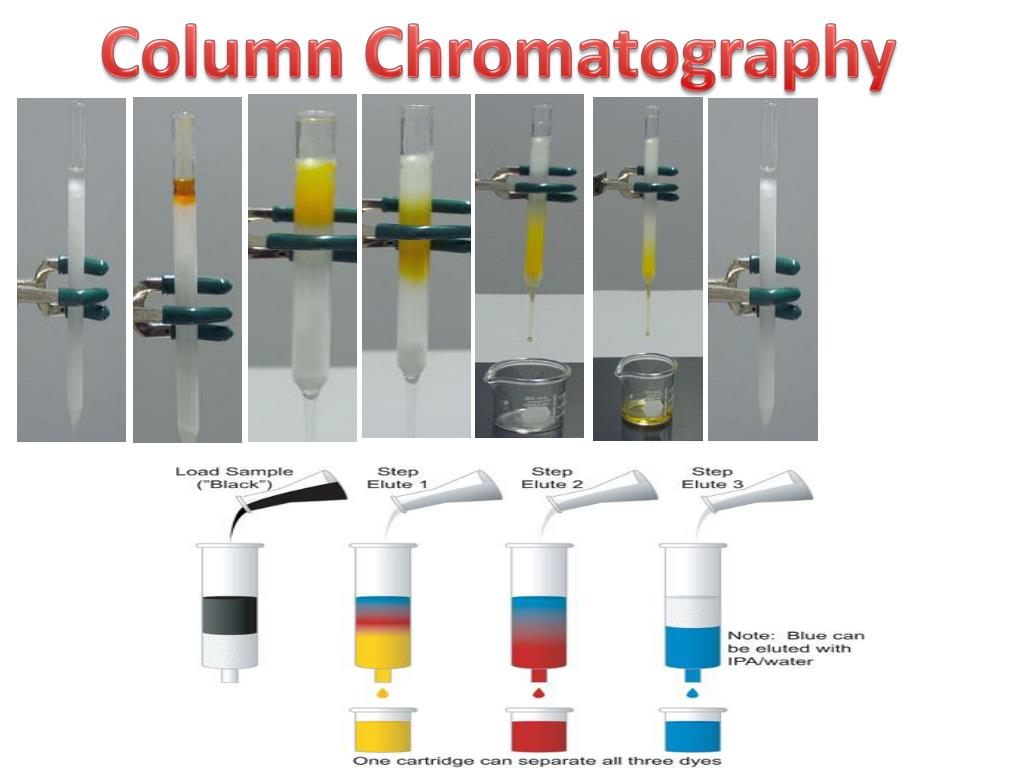
Chromatography Column Qualification . The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Column qualification is performed to assess the integrity and efficiency of a packed. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,.
from www.slideserve.com
Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography is the dominant purification technique in the production of biological compounds, especially for. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. The core document “qualification of equipment” contains the general introduction. Column qualification is performed to assess the integrity and efficiency of a packed.
PPT Column Chromatography PowerPoint Presentation, free download ID
Chromatography Column Qualification Column qualification is performed to assess the integrity and efficiency of a packed. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Column qualification is performed to assess the integrity and efficiency of a packed. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography (lc) equipment qualification process. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. The core document “qualification of equipment” contains the general introduction.
From www.slideshare.net
1.column chromatography Chromatography Column Qualification Chromatography (lc) equipment qualification process. Column qualification is performed to assess the integrity and efficiency of a packed. The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Qualification of chromatographic columns for. Chromatography Column Qualification.
From bitesizebio.com
Column Chromatography Made Simple An Easy to Follow Guide Chromatography Column Qualification This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in the production of biological compounds, especially for. The core document “qualification of equipment”. Chromatography Column Qualification.
From www.chemistrylearner.com
Column Chromatography Definition, Purpose, Types & Applications Chromatography Column Qualification This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Column qualification is performed to assess the integrity and efficiency of a packed. Chromatography (lc) equipment qualification process. The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry.. Chromatography Column Qualification.
From www.slideshare.net
Column chromatography Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification of equipment” contains the general introduction. Column qualification. Chromatography Column Qualification.
From www.studypool.com
SOLUTION Column Chromatography Studypool Chromatography Column Qualification Column qualification is performed to assess the integrity and efficiency of a packed. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in. Chromatography Column Qualification.
From www.scribd.com
Qualification of A Chromatographic Column PDF Chromatography Chromatography Column Qualification The core document “qualification of equipment” contains the general introduction. Column qualification is performed to assess the integrity and efficiency of a packed. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in the production of biological compounds, especially for. This document describes the requirements and general. Chromatography Column Qualification.
From www.slideserve.com
PPT Chromatography and Instrumentation PowerPoint Presentation, free Chromatography Column Qualification Chromatography (lc) equipment qualification process. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. The core document “qualification of equipment” contains the general introduction. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and. Chromatography Column Qualification.
From www.slideserve.com
PPT COLUMN CHROMATOGRAPHY PowerPoint Presentation, free download ID Chromatography Column Qualification This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. The core document “qualification of equipment” contains the general introduction. Column qualification is performed to assess the integrity and efficiency of a packed. Chromatography (lc) equipment qualification process. In the latest installment of biopharm international's element of biopharmaceutical production, five industry.. Chromatography Column Qualification.
From www.studyread.com
Column Chromatography Principle, Procedure & Applications Chromatography Column Qualification Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Column qualification is performed to assess the integrity and efficiency of a packed. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document describes the. Chromatography Column Qualification.
From exopsmrsj.blob.core.windows.net
Column Chromatography Parts at Mary Luna blog Chromatography Column Qualification Chromatography (lc) equipment qualification process. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. The core document “qualification of equipment” contains the general introduction. Column qualification. Chromatography Column Qualification.
From www.bioanalysis-zone.com
What is chromatography and how does it work? Bioanalysis Zone Chromatography Column Qualification Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Chromatography (lc). Chromatography Column Qualification.
From ar.inspiredpencil.com
Column Chromatography Setup Chromatography Column Qualification Chromatography is the dominant purification technique in the production of biological compounds, especially for. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Chromatography (lc) equipment qualification process. Column qualification is performed to assess. Chromatography Column Qualification.
From www.youtube.com
Introduction to Chromatography 7 HPLC Column YouTube Chromatography Column Qualification Chromatography (lc) equipment qualification process. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification of equipment” contains. Chromatography Column Qualification.
From theinstrumentguru.com
Column Chromatography THE INSTRUMENT GURU Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Column qualification is performed to assess the integrity and efficiency of a packed. This document describes the. Chromatography Column Qualification.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Chromatography Column Qualification Chromatography is the dominant purification technique in the production of biological compounds, especially for. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography (lc) equipment qualification process. Column qualification is performed to assess. Chromatography Column Qualification.
From ar.inspiredpencil.com
Column Chromatography Images Chromatography Column Qualification Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Column qualification is performed to assess the integrity and efficiency of a packed. The core document “qualification of equipment” contains the general introduction. Chromatography is the dominant purification technique in the production of biological compounds, especially for. In the. Chromatography Column Qualification.
From bitesizebio.com
The Basics of Running a Chromatography Column Chromatography Column Qualification Chromatography (lc) equipment qualification process. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification of equipment” contains the general introduction. Chromatography is the dominant purification technique in the production of. Chromatography Column Qualification.
From www.slideshare.net
Column chromatography Chromatography Column Qualification Column qualification is performed to assess the integrity and efficiency of a packed. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Chromatography (lc) equipment qualification process. The core document “qualification of equipment” contains the general introduction. In the. Chromatography Column Qualification.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Chromatography Column Qualification The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Column qualification is performed to assess the integrity and efficiency of a packed. Chromatography is the dominant purification technique in the. Chromatography Column Qualification.
From www.studypool.com
SOLUTION Column chromatography Studypool Chromatography Column Qualification Chromatography is the dominant purification technique in the production of biological compounds, especially for. Column qualification is performed to assess the integrity and efficiency of a packed. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification. Chromatography Column Qualification.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Chromatography Column Qualification Column qualification is performed to assess the integrity and efficiency of a packed. The core document “qualification of equipment” contains the general introduction. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document describes the requirements and general criteria. Chromatography Column Qualification.
From scienceinfo.com
Column Chromatography Principle, Instrumentation Chromatography Column Qualification Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Chromatography (lc) equipment qualification process. This document describes the requirements and general criteria for the qualification of. Chromatography Column Qualification.
From mavink.com
Column Chromatography Method Chromatography Column Qualification Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. The core document “qualification of equipment” contains the general introduction. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Column qualification. Chromatography Column Qualification.
From www.researchgate.net
Schematic diagram of column chromatography for the synthesis of Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography (lc) equipment qualification process. Chromatography is the dominant purification technique in the production of biological compounds, especially for. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. The core document “qualification of equipment” contains the general. Chromatography Column Qualification.
From exypldlks.blob.core.windows.net
Hplc Chromatography Method Development at Esther Johnson blog Chromatography Column Qualification Chromatography is the dominant purification technique in the production of biological compounds, especially for. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Column qualification is performed to assess the. Chromatography Column Qualification.
From chembam.com
Biro Column Chromatography Chromatography Column Qualification The core document “qualification of equipment” contains the general introduction. Chromatography is the dominant purification technique in the production of biological compounds, especially for. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns. Chromatography Column Qualification.
From chem.libretexts.org
12.2 General Theory of Column Chromatography Chemistry LibreTexts Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Column qualification is performed to assess the integrity and efficiency of a packed. The core document “qualification of equipment” contains the general introduction. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Chromatography is the dominant purification. Chromatography Column Qualification.
From goldbio.com
How Column Chromatography Works to Separate Proteins GoldBio Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography (lc) equipment qualification process. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Chromatography is the dominant purification technique in. Chromatography Column Qualification.
From pe.agrify.com
Column Chromatography The Definitive Guide Chromatography Column Qualification This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Qualification of chromatographic columns. Chromatography Column Qualification.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography (lc) equipment qualification process. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography is the dominant purification technique in. Chromatography Column Qualification.
From animalia-life.club
Column Gas Chromatography Chromatography Column Qualification The core document “qualification of equipment” contains the general introduction. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Column qualification. Chromatography Column Qualification.
From www.vecteezy.com
Column Chromatography, Separates components based on adsorption and Chromatography Column Qualification In the latest installment of biopharm international's element of biopharmaceutical production, five industry. Chromatography (lc) equipment qualification process. Column qualification is performed to assess the integrity and efficiency of a packed. Chromatography is the dominant purification technique in the production of biological compounds, especially for. The core document “qualification of equipment” contains the general introduction. This document describes the requirements. Chromatography Column Qualification.
From www.slideshare.net
Column chromatography Chromatography Column Qualification Chromatography is the dominant purification technique in the production of biological compounds, especially for. Chromatography (lc) equipment qualification process. Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. The core document “qualification of equipment” contains the general introduction. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. This document. Chromatography Column Qualification.
From www.studocu.com
Type of column chromatography ) Stationary Phase in column Chromatography Column Qualification Qualification of chromatographic columns for analytical testing in biologics and develop solutions and performance standards to. Chromatography (lc) equipment qualification process. This document describes the requirements and general criteria for the qualification of analytical columns used in liquid chromatography (lc,. Column qualification is performed to assess the integrity and efficiency of a packed. The core document “qualification of equipment” contains. Chromatography Column Qualification.
From scienceinfo.com
Column Chromatography Principle, Instrumentation Chromatography Column Qualification Chromatography (lc) equipment qualification process. In the latest installment of biopharm international's element of biopharmaceutical production, five industry. The core document “qualification of equipment” contains the general introduction. Chromatography is the dominant purification technique in the production of biological compounds, especially for. Column qualification is performed to assess the integrity and efficiency of a packed. This document describes the requirements. Chromatography Column Qualification.