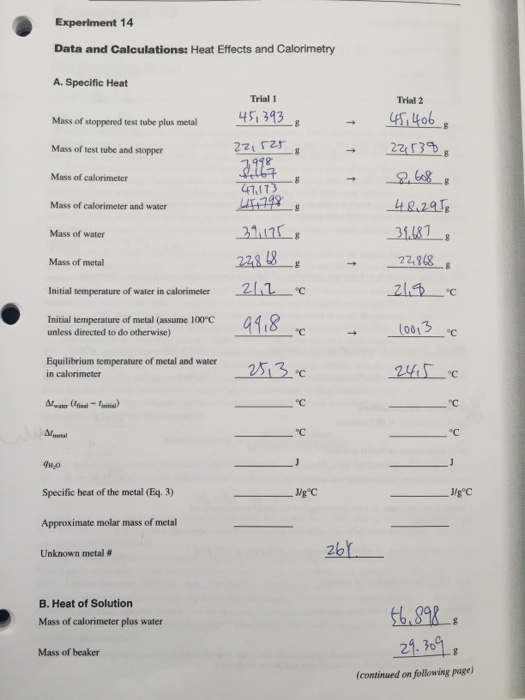
Heat Effects And Calorimetry Lab Report . Lab session 9, experiment 8: Calorimetry is used to measure amounts of heat. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Heat is a form of energy that is. Heat is a form of energy, sometimes called thermal energy, which can. Introduction heat is a form of energy, which can flow between objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Determine the specific heat capacity of a metal using a coffee cup calorimeter.
from cityraven.com
Calorimetry is used to measure amounts of heat. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Introduction heat is a form of energy, which can flow between objects. Heat is a form of energy that is. Heat is a form of energy, sometimes called thermal energy, which can. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Lab session 9, experiment 8:
👍 Heat effects and calorimetry lab answers. Heat Effects And
Heat Effects And Calorimetry Lab Report Heat is a form of energy, sometimes called thermal energy, which can. Heat is a form of energy, sometimes called thermal energy, which can. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Lab session 9, experiment 8: Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Introduction heat is a form of energy, which can flow between objects. Heat is a form of energy that is. Calorimetry is used to measure amounts of heat.
From www.chegg.com
Mass of calorimeter Mass of calorimeter and water Heat Effects And Calorimetry Lab Report Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Heat is a form of energy, sometimes called thermal energy, which can. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or. Heat Effects And Calorimetry Lab Report.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Heat Effects And Calorimetry Lab Report Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Heat is a form. Heat Effects And Calorimetry Lab Report.
From www.chegg.com
Solved Heat Effects And Calorimetry Lab Report with data Heat Effects And Calorimetry Lab Report Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Heat is a form of energy that is. Lab session 9, experiment 8: Calorimetry is used to measure amounts of heat. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Introduction. Heat Effects And Calorimetry Lab Report.
From studylib.net
Lab Session 9, Experiment 8 Calorimetry, Heat of Reaction Heat Effects And Calorimetry Lab Report Introduction heat is a form of energy, which can flow between objects. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Lab session 9, experiment 8: Heat is a form of energy that is. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Chem 17.1 Exp. 2 Experiment 2 HEAT EFFECTS AND CALORIMETRY A Heat Effects And Calorimetry Lab Report Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Heat. Heat Effects And Calorimetry Lab Report.
From www.studypool.com
SOLUTION Online experiment heat effects and calorimetry Studypool Heat Effects And Calorimetry Lab Report Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Introduction heat is a form of energy, which can flow between objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is. Heat Effects And Calorimetry Lab Report.
From www.chegg.com
106 Experiment 14 Heat Effects and Calorimetry d. Heat Effects And Calorimetry Lab Report Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Lab session 9, experiment 8: Heat is measured. Heat Effects And Calorimetry Lab Report.
From www.coursehero.com
[Solved] Experiment 7 Heat Effects and Calorimetry PreLaboratory Heat Effects And Calorimetry Lab Report Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is used to measure amounts of heat. Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. Heat Effects And Calorimetry Lab Report.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Heat Effects And Calorimetry Lab Report Heat is a form of energy, sometimes called thermal energy, which can. Lab session 9, experiment 8: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Heat Effects And Calorimetry Lab Report.
From www.chegg.com
Experiment 14 Data and Calculations Heat Effects and Heat Effects And Calorimetry Lab Report Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Calorimetry, heat of reaction specific. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Laboratory report Heat Effects and Calorimetry Name Date Performed Heat Effects And Calorimetry Lab Report Heat is a form of energy that is. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Assuming that no heat is lost. Heat Effects And Calorimetry Lab Report.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Heat Effects And Calorimetry Lab Report Lab session 9, experiment 8: Calorimetry is used to measure amounts of heat. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Heat Effects And Calorimetry Lab Report Heat is a form of energy that is. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Lab session 9, experiment 8: Introduction heat is a form of energy, which can flow between objects. Calorimetry, heat of reaction specific heat is an intensive property. Heat Effects And Calorimetry Lab Report.
From www.bartleby.com
Answered Experiment 14 Data and Calculations… bartleby Heat Effects And Calorimetry Lab Report Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is a form of energy that. Heat Effects And Calorimetry Lab Report.
From www.coursehero.com
[Solved] Heat Effects And Calorimetry Lab Report with data Table 1 Heat Effects And Calorimetry Lab Report Lab session 9, experiment 8: Calorimetry is used to measure amounts of heat. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls,. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
P calorimetry 25 lab report StuDocu Heat Effects And Calorimetry Lab Report Heat is a form of energy that is. Calorimetry is used to measure amounts of heat. Introduction heat is a form of energy, which can flow between objects. Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Heat is. Heat Effects And Calorimetry Lab Report.
From studylib.net
06.03 Calorimetry Lab Report Heat Effects And Calorimetry Lab Report Lab session 9, experiment 8: Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Heat is measured in the energy units, joules (j), calorimetry is the. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Lab Manual 5 Heat Effects and Calorimetry Lab Report Experiment 14 Heat Effects And Calorimetry Lab Report Introduction heat is a form of energy, which can flow between objects. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat is. Heat Effects And Calorimetry Lab Report.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Heat Effects And Calorimetry Lab Report Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Introduction heat is a form of energy, which can flow between objects. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a. Heat Effects And Calorimetry Lab Report.
From cityraven.com
🎉 Heat effects and calorimetry lab. Calorimeter. 20190203 Heat Effects And Calorimetry Lab Report Heat is a form of energy, sometimes called thermal energy, which can. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold. Heat Effects And Calorimetry Lab Report.
From www.scribd.com
2 Heat Effects and Calorimetry PDF Calorimetry Enthalpy Heat Effects And Calorimetry Lab Report One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Introduction heat is a form of energy, which can flow between objects. Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool. Heat Effects And Calorimetry Lab Report.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Heat Effects And Calorimetry Lab Report Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Calorimetry. Heat Effects And Calorimetry Lab Report.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Heat Effects And Calorimetry Lab Report Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Heat is measured in the energy. Heat Effects And Calorimetry Lab Report.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Thinkswap Heat Effects And Calorimetry Lab Report Heat is a form of energy, sometimes called thermal energy, which can. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Heat is measured in the energy units, joules (j), calorimetry is the study. Heat Effects And Calorimetry Lab Report.
From www.scribd.com
Lab 07Specific Heat & Calorimetry.pdf Heat Temperature Heat Effects And Calorimetry Lab Report Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: Heat is a form of energy, sometimes called thermal energy, which can. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Copy of Lab 5 Calorimetry and Specific Heat Lab Calorimetry and Heat Effects And Calorimetry Lab Report Calorimetry is used to measure amounts of heat. Heat is a form of energy that is. Lab session 9, experiment 8: Heat is a form of energy, sometimes called thermal energy, which can. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Introduction heat. Heat Effects And Calorimetry Lab Report.
From studylib.net
Calorimetry Lab Specific Heat Capacity Heat Effects And Calorimetry Lab Report Determine the specific heat capacity of a metal using a coffee cup calorimeter. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Heat. Heat Effects And Calorimetry Lab Report.
From cityraven.com
👍 Heat effects and calorimetry lab answers. Heat Effects And Heat Effects And Calorimetry Lab Report Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat. Heat Effects And Calorimetry Lab Report.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Heat Effects And Calorimetry Lab Report Heat is measured in the energy units, joules (j), calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Heat is a form of energy, sometimes called thermal energy, which can. Calorimetry is used to measure amounts of heat. Experiment 6 ∙ calorimetry 6‐3 consider what happens when. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Heat Effects and Calorimetry Lab Report Name Akzhan Yestoreyeva Date Heat Effects And Calorimetry Lab Report Introduction heat is a form of energy, which can flow between objects. Lab session 9, experiment 8: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity. Heat Effects And Calorimetry Lab Report.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Heat Effects And Calorimetry Lab Report Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside. Heat Effects And Calorimetry Lab Report.
From humberto.perka.org
Experiment 14 Heat Effects And Calorimetry Advance Study Assignment Heat Effects And Calorimetry Lab Report Heat is a form of energy that is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter.. Heat Effects And Calorimetry Lab Report.
From www.studypool.com
SOLUTION Online experiment heat effects and calorimetry Studypool Heat Effects And Calorimetry Lab Report One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity of hot water is poured into a quantity of cold water inside a calorimeter. Calorimetry is used to measure amounts of heat. Heat is a form of. Heat Effects And Calorimetry Lab Report.
From www.chegg.com
Experiment 14 Data and Calculations Heat Effects and Heat Effects And Calorimetry Lab Report Calorimetry is used to measure amounts of heat. Heat is a form of energy, sometimes called thermal energy, which can. Assuming that no heat is lost from the calorimeter to the surroundings, and that a negligible amount of heat is absorbed by the calorimeter walls, the amount of heat that. Heat is measured in the energy units, joules (j), calorimetry. Heat Effects And Calorimetry Lab Report.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Heat Effects And Calorimetry Lab Report Heat is a form of energy, sometimes called thermal energy, which can. Determine the specific heat capacity of a metal using a coffee cup calorimeter. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Experiment 6 ∙ calorimetry 6‐3 consider what happens when a quantity. Heat Effects And Calorimetry Lab Report.