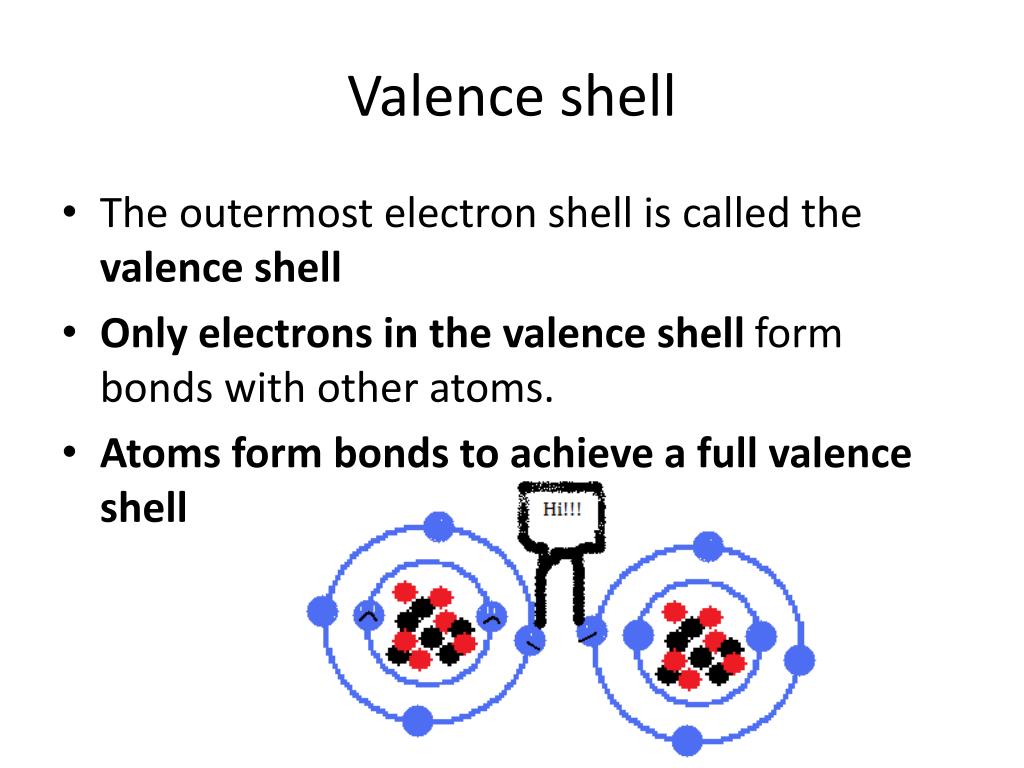
Define Expanded Valence Shell . More common than incomplete octets are expanded octets where the central atom in a lewis. Write the chemical formulas and chemical names of covalent molecules that contain atoms. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Such compounds are formed only by central atoms in the third row of the periodic. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. These are called expanded valence shell molecules. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. Identify which elements can expand their valences when forming covalent molecules.
from www.slideserve.com
Such compounds are formed only by central atoms in the third row of the periodic. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. These are called expanded valence shell molecules. Write the chemical formulas and chemical names of covalent molecules that contain atoms. Identify which elements can expand their valences when forming covalent molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. More common than incomplete octets are expanded octets where the central atom in a lewis.
PPT Atomic structure and chemical bonding PowerPoint Presentation
Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. Such compounds are formed only by central atoms in the third row of the periodic. Write the chemical formulas and chemical names of covalent molecules that contain atoms. These are called expanded valence shell molecules. Identify which elements can expand their valences when forming covalent molecules. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. More common than incomplete octets are expanded octets where the central atom in a lewis. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding.
From www.slideserve.com
PPT Exceptions to the Octet Rule PowerPoint Presentation, free Define Expanded Valence Shell More common than incomplete octets are expanded octets where the central atom in a lewis. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and chemical names of covalent molecules that contain atoms. Such compounds are formed only by central atoms in the third row of. Define Expanded Valence Shell.
From byjus.com
Different energy shells, valence shell, valence electrons Define Expanded Valence Shell These are called expanded valence shell molecules. Write the chemical formulas and chemical names of covalent molecules that contain atoms. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule. Define Expanded Valence Shell.
From www.slideserve.com
PPT Lecture 24 Beyond the Octet PowerPoint Presentation, free Define Expanded Valence Shell Such compounds are formed only by central atoms in the third row of the periodic. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. More common than incomplete octets are expanded octets where the central atom in a lewis. The number of electron pairs in the valence shell. Define Expanded Valence Shell.
From www.slideserve.com
PPT Chapter 9 Molecular Geometries and Bonding Theories PowerPoint Define Expanded Valence Shell These are called expanded valence shell molecules. Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. In chemistry, a hypervalent molecule (the. Define Expanded Valence Shell.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. More common than incomplete octets are expanded octets where the central atom in a lewis. Such compounds are formed only by central atoms in the third row of the periodic.. Define Expanded Valence Shell.
From www.youtube.com
Expanded Valence Shell Bonding YouTube Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Write the chemical formulas and chemical names of covalent molecules that contain atoms. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of. Define Expanded Valence Shell.
From www.expii.com
Valence Electrons — Definition & Importance Expii Define Expanded Valence Shell In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. More common than incomplete octets are expanded octets where the central atom in a lewis. These are called expanded valence shell molecules. Identify which elements can expand their valences when forming covalent molecules. The number of electron pairs in the valence. Define Expanded Valence Shell.
From slideplayer.com
Valence and Core Electrons ppt download Define Expanded Valence Shell These are called expanded valence shell molecules. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. The number of electron pairs in the valence shell of a central atom. Define Expanded Valence Shell.
From www.slideserve.com
PPT Chap. 4 Chemical Bonding PowerPoint Presentation, free download Define Expanded Valence Shell These are called expanded valence shell molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. Write the chemical formulas and chemical names. Define Expanded Valence Shell.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. These are called expanded valence shell molecules. Such compounds are formed only by central atoms in the third row of the periodic. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. The number of electron pairs in. Define Expanded Valence Shell.
From studylib.net
Expanded valence shells (extended octets) Define Expanded Valence Shell Write the chemical formulas and chemical names of covalent molecules that contain atoms. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. The number of electron pairs in the. Define Expanded Valence Shell.
From www.slideserve.com
PPT Molecular Geometry PowerPoint Presentation ID2422169 Define Expanded Valence Shell Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. More common than incomplete octets are expanded octets where the central atom in a lewis. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell Such compounds are formed only by central atoms in the third row of the periodic. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Molecules. Define Expanded Valence Shell.
From www.slideserve.com
PPT Resonance Structures PowerPoint Presentation, free download ID Define Expanded Valence Shell Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. These are called expanded valence shell molecules. Write the chemical formulas and chemical names. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Such compounds are formed only by central atoms in the third row of the periodic. Write the chemical formulas and chemical names of covalent molecules that contain atoms. In chemistry, a hypervalent molecule (the. Define Expanded Valence Shell.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID4510494 Define Expanded Valence Shell These are called expanded valence shell molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Write the chemical formulas and chemical names of covalent molecules that contain atoms. More common than incomplete octets are expanded octets where the central atom in a lewis.. Define Expanded Valence Shell.
From www.slideserve.com
PPT Semiconductor Basics PowerPoint Presentation, free download ID Define Expanded Valence Shell Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. These are called expanded valence shell molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Such compounds are formed only by central atoms in the third row of the periodic. In chemistry,. Define Expanded Valence Shell.
From slideplayer.com
Chapter 11 Chemical Bonding I Basic Concepts ppt download Define Expanded Valence Shell In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. These are called expanded valence shell molecules. Such compounds are formed only by central atoms. Define Expanded Valence Shell.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free Define Expanded Valence Shell Write the chemical formulas and chemical names of covalent molecules that contain atoms. Identify which elements can expand their valences when forming covalent molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Such compounds are formed only by central atoms in the third. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Identify which elements can expand their valences when forming covalent molecules. Such compounds are formed only by central atoms in the third row of the periodic. Write the chemical formulas and chemical names of covalent molecules that contain atoms. The number. Define Expanded Valence Shell.
From www.numerade.com
SOLVED To represent the right Lewis structure of one of these Define Expanded Valence Shell Write the chemical formulas and chemical names of covalent molecules that contain atoms. Such compounds are formed only by central atoms in the third row of the periodic. Identify which elements can expand their valences when forming covalent molecules. These are called expanded valence shell molecules. The number of electron pairs in the valence shell of a central atom is. Define Expanded Valence Shell.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Define Expanded Valence Shell The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. These are called expanded valence shell molecules. Such compounds are formed only by central atoms in. Define Expanded Valence Shell.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. The number of electron pairs in the valence shell of a central atom is determined. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell More common than incomplete octets are expanded octets where the central atom in a lewis. These are called expanded valence shell molecules. Identify which elements can expand their valences when forming covalent molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Molecules with. Define Expanded Valence Shell.
From www.expii.com
Valence Electrons — Definition & Importance Expii Define Expanded Valence Shell In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. These are called expanded valence shell molecules. Write the chemical formulas and chemical names of covalent molecules that contain atoms. Such compounds are formed only by central atoms in the third row of the periodic. Molecules with expanded valence shells in. Define Expanded Valence Shell.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary Define Expanded Valence Shell In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with. Define Expanded Valence Shell.
From www.youtube.com
Structural Chemistry I, Video IV Expanded Valence Shells YouTube Define Expanded Valence Shell Such compounds are formed only by central atoms in the third row of the periodic. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. More common than incomplete octets are expanded octets where the central atom in a lewis. These are called expanded valence shell molecules. Write the. Define Expanded Valence Shell.
From www.slideserve.com
PPT Atomic structure and chemical bonding PowerPoint Presentation Define Expanded Valence Shell More common than incomplete octets are expanded octets where the central atom in a lewis. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and chemical names of covalent molecules that contain atoms. Such compounds are formed only by central atoms in the third row of. Define Expanded Valence Shell.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID2453814 Define Expanded Valence Shell These are called expanded valence shell molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Identify which elements can expand their valences when forming covalent molecules. Write the chemical formulas and chemical names of covalent molecules that contain atoms. Such compounds are formed. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell More common than incomplete octets are expanded octets where the central atom in a lewis. Molecules with expanded valence shells in chemistry and physics, an expanded octet is a molecule with an electron configuration that is. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Write the chemical formulas and. Define Expanded Valence Shell.
From www.youtube.com
VSEPR for Expanded Valence Shell YouTube Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Such compounds are formed only by central atoms in the third row of the periodic. The number of electron pairs in the valence shell of a central atom is determined. Define Expanded Valence Shell.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Define Expanded Valence Shell In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains. Such compounds are formed only by central atoms in the third row of the periodic. Identify which elements can expand their valences when forming covalent molecules. The number of electron pairs in the valence shell of a central atom is determined. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell These are called expanded valence shell molecules. The number of electron pairs in the valence shell of a central atom is determined after drawing the lewis structure of the molecule, and expanding. Write the chemical formulas and chemical names of covalent molecules that contain atoms. Such compounds are formed only by central atoms in the third row of the periodic.. Define Expanded Valence Shell.
From www.numerade.com
SOLVEDWhich elements can form expanded valence shell molecules? Do the Define Expanded Valence Shell Identify which elements can expand their valences when forming covalent molecules. These are called expanded valence shell molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Such compounds are formed only by central atoms in the third row of the periodic. Molecules with expanded valence shells in chemistry and physics, an expanded octet. Define Expanded Valence Shell.
From www.sliderbase.com
Molecules with Expanded Valence Shells Define Expanded Valence Shell Such compounds are formed only by central atoms in the third row of the periodic. These are called expanded valence shell molecules. More common than incomplete octets are expanded octets where the central atom in a lewis. Identify which elements can expand their valences when forming covalent molecules. Molecules with expanded valence shells in chemistry and physics, an expanded octet. Define Expanded Valence Shell.