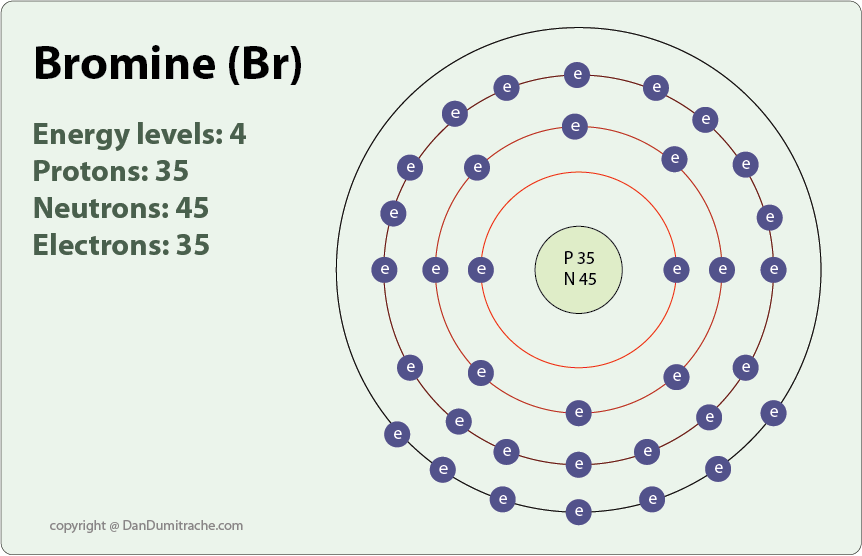
Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond . Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Bromine has 7 valence electrons and is in group 17 of the periodic table. Find out the valency of bromine in different states. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. The valency of bromine is 1, meaning it can form one covalent bond, while the. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Valence electrons found in the s and p orbitals of the highest energy. In the case of dibromine, the combined number of.
from ar.inspiredpencil.com
Bromine has 7 valence electrons and is in group 17 of the periodic table. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Find out the valency of bromine in different states. In the case of dibromine, the combined number of. Valence electrons found in the s and p orbitals of the highest energy. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. The valency of bromine is 1, meaning it can form one covalent bond, while the.
Bromine Valence Electrons
Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons of bromine using periodic table or electron configuration. Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. In the case of dibromine, the combined number of. Find out the valency of bromine in different states. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Learn how to find the valence electrons of bromine using periodic table or electron configuration. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Valence electrons found in the s and p orbitals of the highest energy. Bromine has 7 valence electrons and is in group 17 of the periodic table. The valency of bromine is 1, meaning it can form one covalent bond, while the.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Find out the valency of bromine in different states. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. 93 rows learn the valences of the chemical elements, the number of electrons. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond The valency of bromine is 1, meaning it can form one covalent bond, while the. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. In the case of dibromine, the combined number of. Learn how to find the valence electrons of bromine using periodic table or electron. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.youtube.com
Electron Configuration of Bromine, Br YouTube Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has 7 valence electrons and is in group 17 of the periodic table. Valence electrons found in the s and p orbitals of the highest energy. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. In the case of dibromine, the combined number of. Find out. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From ar.inspiredpencil.com
Bromine Valence Electrons Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has 7 valence electrons and is in group 17 of the periodic table. In the case of dibromine, the combined number of. Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. 93 rows learn the valences of the chemical elements,. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Learn how to find the valence electrons of bromine using periodic table or electron configuration. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. In the. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.dreamstime.com
Bromine Chemical 35 Element of Periodic Table. Molecule and Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Valence electrons found in the s and p orbitals of the highest energy. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Find out the valency of bromine in. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has 7 valence electrons and is in group 17 of the periodic table. The valency of bromine is 1, meaning it can form one covalent bond, while the. In the case of dibromine, the combined number of. Find out the valency of bromine in different states. Learn how to draw the lewis structure of bromine molecule (br2), its molecular. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.numerade.com
SOLVED The compound below is treated with Nbromosuccinimide (NBS) in Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has 7 valence electrons and is in group 17 of the periodic table. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. In the case of dibromine, the combined number of. Learn how to draw. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.alamyimages.fr
Schéma d'électrons et de symbole pour le brome illustration Image Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons of bromine using periodic table or electron configuration. In the case of dibromine, the combined number of. Find out the valency of bromine in different states. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Valence electrons found in the s. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Valence electrons found in the s and p orbitals of the highest energy. The valency of bromine is 1, meaning it can form one covalent bond, while the. As we know that bromine lies in the same group as chlorine and fluorine,. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From phootoscelebrities.com
How Many Valence Electrons Does Bromine Have? Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Valence electrons found in the s and p orbitals of the highest energy. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.vrogue.co
Periodic Table With Valence Electrons Free Printable vrogue.co Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The valency of bromine is 1, meaning it can form one covalent bond, while the. In the case of. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From srkrkzbhxtdlh.blogspot.com
How Many Valence Electrons Does Bromine Have Bromine is a group viia Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons of bromine using periodic table or electron configuration. Bromine has 7 valence electrons and is in group 17 of the periodic table. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. The valency of bromine is 1, meaning it can form. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. The valency of bromine is 1, meaning it can form one covalent bond, while the. Learn how to find the valence electrons of bromine. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From answerlibcottise.z21.web.core.windows.net
What Element Group Has 7 Valence Electrons Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Bromine has 7 valence electrons and is in group 17 of the periodic table. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Find out the valency of bromine in different states. The valency of bromine is 1, meaning it can. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Valence electrons found in the s and p orbitals of the highest energy. Bromine has 7 valence electrons and is in group 17 of the periodic table. Find out the valency of bromine in different states. As we know that bromine lies. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond In the case of dibromine, the combined number of. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. The valency of bromine is 1, meaning it can form one covalent bond, while the. Find out the valency of bromine in different states. Learn how to draw the. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From bromineinformation.weebly.com
Bromine on the Periodic Table Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Find out the valency of bromine in different states. Valence electrons found in the s and p orbitals of the highest energy. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.numerade.com
The compound below is treated with Nbromosuccinimide (NBS) in the Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Valence electrons found in the s and p orbitals of the highest energy. As we know that bromine lies in the same group as chlorine and fluorine,. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Valence electrons found in the. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons of bromine using periodic table or electron configuration. Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. In the case of. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From proper-cooking.info
Bromine Valence Electrons Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond The valency of bromine is 1, meaning it can form one covalent bond, while the. Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. As we know that bromine lies. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.americanelements.com
Bromine (Br) AMERICAN ELEMENTS Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond The valency of bromine is 1, meaning it can form one covalent bond, while the. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Bromine has 7 valence electrons and is in group 17 of the. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Valence electrons found in the s and p orbitals of the highest energy. Bromine has 7 valence electrons and is in group 17 of the periodic table. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Find out the valency of bromine in different states. Learn how. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Find out the valency of bromine in different states. Bromine has 7 valence electrons and is in group 17 of the periodic table. Valence electrons found in the s and p orbitals of the highest energy. Learn how to. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From periodictable.me
How Do We Find The Electron Configuration For Bromine Dynamic Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Valence electrons found in the s and p orbitals of the highest energy. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. Bromine has 7 valence electrons and is in group 17 of the periodic table. Learn how to find the valence electrons of bromine using periodic table. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From chromefity.weebly.com
Find valence electrons on periodic table chromefity Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond The valency of bromine is 1, meaning it can form one covalent bond, while the. Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. The valency of bromine is 1, meaning it can form one covalent bond, while the. In the case of dibromine, the combined number of. Find out the valency of bromine in different states. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From ar.inspiredpencil.com
Bromine Electron Dot Diagram Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Valence electrons found in the s and p orbitals of the highest energy. Find out the valency of bromine in different states. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond or form. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons and valency of bromine, a reactive halogen with atomic number 35. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Learn how to find the valence electrons of bromine using periodic table or electron configuration. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.thoughtco.com
Color Periodic Table of the Elements Valence Charge Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to find the valence electrons of bromine using periodic table or electron configuration. Valence electrons found in the s and p orbitals of the highest energy. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. In the case of dibromine, the combined number of. Bromine. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. Learn how to find the valence electrons of bromine using periodic table or electron configuration. Find out the valency of bromine in different states. Valence electrons found in the s and p orbitals of the highest energy. Learn how to draw the lewis structure of bromine molecule (br2), its molecular. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From printablelibbrained.z13.web.core.windows.net
What Shows The Number Of Valence Electrons Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Find out the valency of bromine in different states. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. 93 rows learn the valences of the chemical elements, the number of electrons with which an atom will bond. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond Learn how to draw the lewis structure of bromine molecule (br2), its molecular geometry, hybridisation, polarity and faqs. Bromine has 7 valence electrons and is in group 17 of the periodic table. Bromine has an electron configuration of 1s^2 2s^2 2p^6 3s^2. As we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons. Bromine Has 7 Valence Electrons. With What Group Of Elements Will Bromine Most Readily Bond.