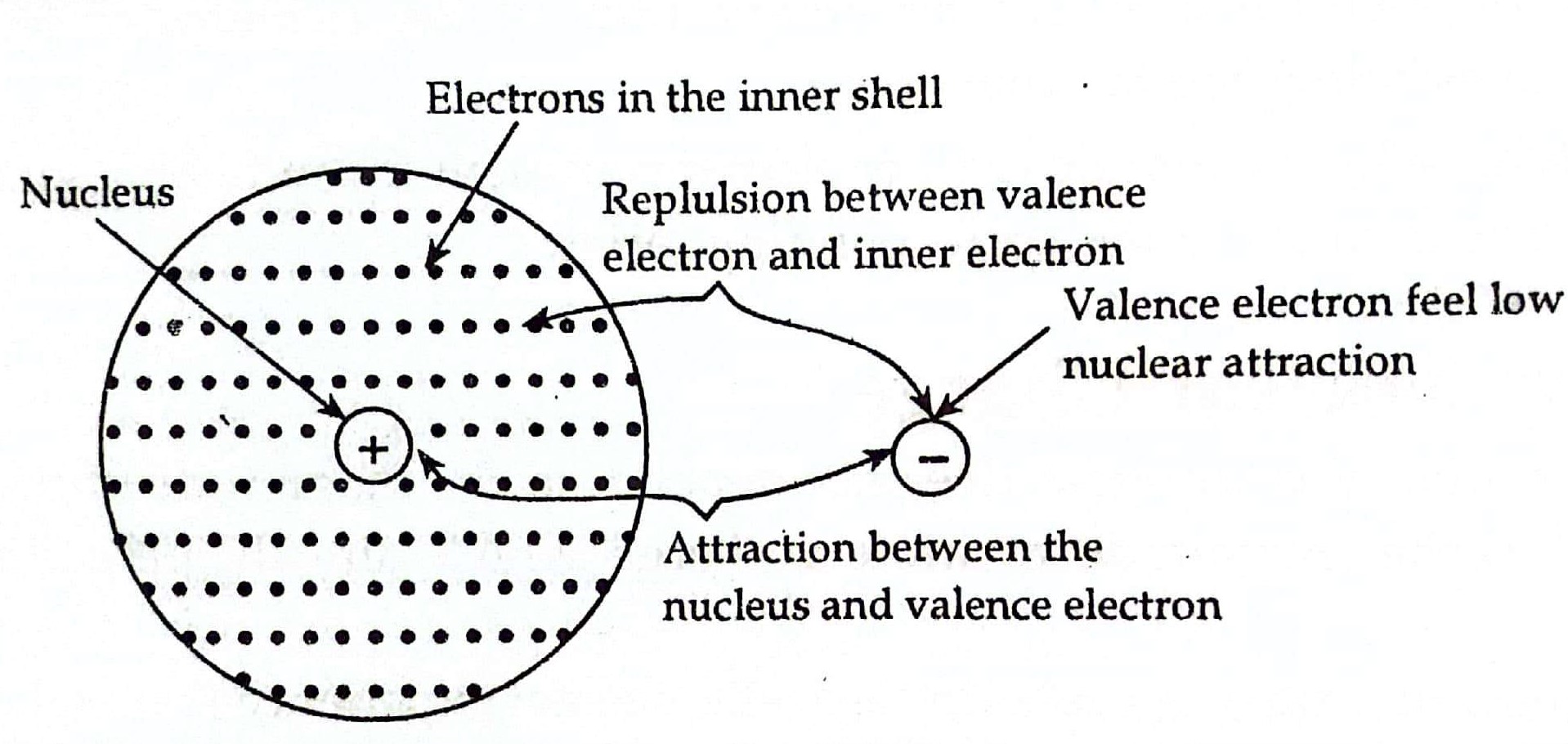
Electron Shielding Effect Size . Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. See how effective nuclear charge (zeff) varies. Learn how atomic radii are defined and measured for different types of bonds and elements. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. Explore the factors that affect the size of atoms and.
from chemistnotes.com
The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Explore the factors that affect the size of atoms and. See how effective nuclear charge (zeff) varies. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how atomic radii are defined and measured for different types of bonds and elements. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons.
Shielding Effect or Screening Effect Definition, Factors Affecting
Electron Shielding Effect Size The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. Explore the factors that affect the size of atoms and. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how atomic radii are defined and measured for different types of bonds and elements. See how effective nuclear charge (zeff) varies.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Explore the factors that affect the size of atoms and. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. See how effective nuclear charge (zeff) varies. The shielding effect. Electron Shielding Effect Size.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Shielding Effect Size See how effective nuclear charge (zeff) varies. Explore the factors that affect the size of atoms and. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn. Electron Shielding Effect Size.
From surfguppy.com
What is Electronegativity? Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how atomic radii are defined and measured for different types of bonds and elements. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. See how effective nuclear charge. Electron Shielding Effect Size.
From scienceinfo.com
Shielding effect Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest. Electron Shielding Effect Size.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.1 Electronegativity翰林国际教育 Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how atomic radii are defined and measured for different types of bonds and elements. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect describes the balance between the pull of the protons on valence electrons. Electron Shielding Effect Size.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.1.2 Electron Affinity & Trends of Group 16 Electron Shielding Effect Size The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. See how effective nuclear charge (zeff) varies. Learn how atomic radii are defined and measured for different types of bonds and elements. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron. Electron Shielding Effect Size.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint Electron Shielding Effect Size Learn how atomic radii are defined and measured for different types of bonds and elements. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. See how. Electron Shielding Effect Size.
From www.slideshare.net
Periodic trends Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how electron shielding. Electron Shielding Effect Size.
From chemistry.stackexchange.com
chemistry electron electron repulsion and shielding Electron Shielding Effect Size Learn how atomic radii are defined and measured for different types of bonds and elements. See how effective nuclear charge (zeff) varies. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. Explore the factors that affect the size of atoms and. Learn how electrons shield each other from. Electron Shielding Effect Size.
From byjus.com
What is shielding and deshielding in NMR? Give an example? Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. See how effective nuclear charge (zeff) varies. The shielding effect is shown by the interior electron cloud (light blue) shielding. Electron Shielding Effect Size.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2617702 Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. Learn how atomic radii are defined and measured for different types of bonds and elements. The shielding effect describes the. Electron Shielding Effect Size.
From chemizi.blogspot.com
Actinide contractiondefinitioncausesconsequences in chemistry Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect is shown by the interior electron. Electron Shielding Effect Size.
From mungfali.com
What Is Shielding Effect Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from. Electron Shielding Effect Size.
From www.numerade.com
SOLVED The diagram below shows the relative atomic sizes of three Electron Shielding Effect Size The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. See how effective nuclear charge (zeff) varies. Explore the factors that affect the size. Electron Shielding Effect Size.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The Electron Shielding Effect Size Learn how atomic radii are defined and measured for different types of bonds and elements. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. The shielding effect describes. Electron Shielding Effect Size.
From ar.inspiredpencil.com
Shielding Effect Electrons Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the. Electron Shielding Effect Size.
From mungfali.com
What Is Shielding Effect Electron Shielding Effect Size The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. See how effective nuclear charge (zeff) varies. Learn how core electrons shield. Electron Shielding Effect Size.
From animalia-life.club
Shielding Effect Trend Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer. Electron Shielding Effect Size.
From ecurrencythailand.com
What Is The Electron Shielding Effect? Best 7 Answer Electron Shielding Effect Size The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest. Electron Shielding Effect Size.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. See how effective nuclear charge (zeff) varies. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Explore the factors that affect the size. Electron Shielding Effect Size.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID6734591 Electron Shielding Effect Size Learn how atomic radii are defined and measured for different types of bonds and elements. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces. Electron Shielding Effect Size.
From chemisfast.blogspot.com
Lanthanide contractiondefinitioncausesconsequences in chemistry PG Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how atomic radii. Electron Shielding Effect Size.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. See how effective nuclear charge (zeff) varies. The shielding effect is shown by the interior electron cloud (light. Electron Shielding Effect Size.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. See how effective nuclear charge (zeff) varies. Learn. Electron Shielding Effect Size.
From www.chem.fsu.edu
Electron Configurations Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. The shielding effect describes the balance between the pull of the protons on valence. Electron Shielding Effect Size.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how atomic radii are defined and measured for different types of bonds and elements. See how effective nuclear charge (zeff) varies. Explore the factors that affect the size of atoms and. The shielding effect is shown by the interior. Electron Shielding Effect Size.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity Electron Shielding Effect Size The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how atomic radii are defined and measured for different types of bonds and elements. Explore the factors that affect the size of atoms and. See how effective nuclear charge (zeff) varies. The shielding effect is shown by. Electron Shielding Effect Size.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect describes the balance between the pull of. Electron Shielding Effect Size.
From socratic.org
How are shielding effect and atomic radius related? Socratic Electron Shielding Effect Size The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. See how effective nuclear charge (zeff) varies. Learn how core electrons shield. Electron Shielding Effect Size.
From ar.inspiredpencil.com
Electron Shielding Electron Shielding Effect Size Explore the factors that affect the size of atoms and. Learn how atomic radii are defined and measured for different types of bonds and elements. Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. Learn how electron shielding reduces the attractive interaction between an electron and the nucleus,. Electron Shielding Effect Size.
From www.echemi.com
My book's claim about the shielding effect of s,p,d and f electrons Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. See how effective nuclear charge (zeff) varies. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how electron shielding reduces the attractive. Electron Shielding Effect Size.
From slideplayer.com
Chemical Periodicity Chapter ppt download Electron Shielding Effect Size Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Explore the factors that affect the size of atoms and. Learn how atomic radii are defined and measured for different types of bonds and elements. The shielding effect describes the balance between the pull of the protons on valence electrons. Electron Shielding Effect Size.
From animalia-life.club
Shielding Effect Trend Electron Shielding Effect Size Learn how core electrons shield outer electrons from the nuclear charge and how this affects the periodic properties of the elements. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest from the full attractive force of the nucleus. Learn how atomic radii are defined and measured for different types of bonds. Electron Shielding Effect Size.
From www.learner.org
Organizing Atoms and Electrons The Periodic Table Annenberg Learner Electron Shielding Effect Size Explore the factors that affect the size of atoms and. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. Learn how electrons shield each other from the nucleus and affect the effective nuclear charge (zeff) experienced by outer electrons. Learn how core electrons shield outer electrons from. Electron Shielding Effect Size.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting Electron Shielding Effect Size Learn how electron shielding reduces the attractive interaction between an electron and the nucleus, and how to calculate the effective. The shielding effect describes the balance between the pull of the protons on valence electrons and the repulsion forces from inner electrons. The shielding effect is shown by the interior electron cloud (light blue) shielding the outer electron of interest. Electron Shielding Effect Size.