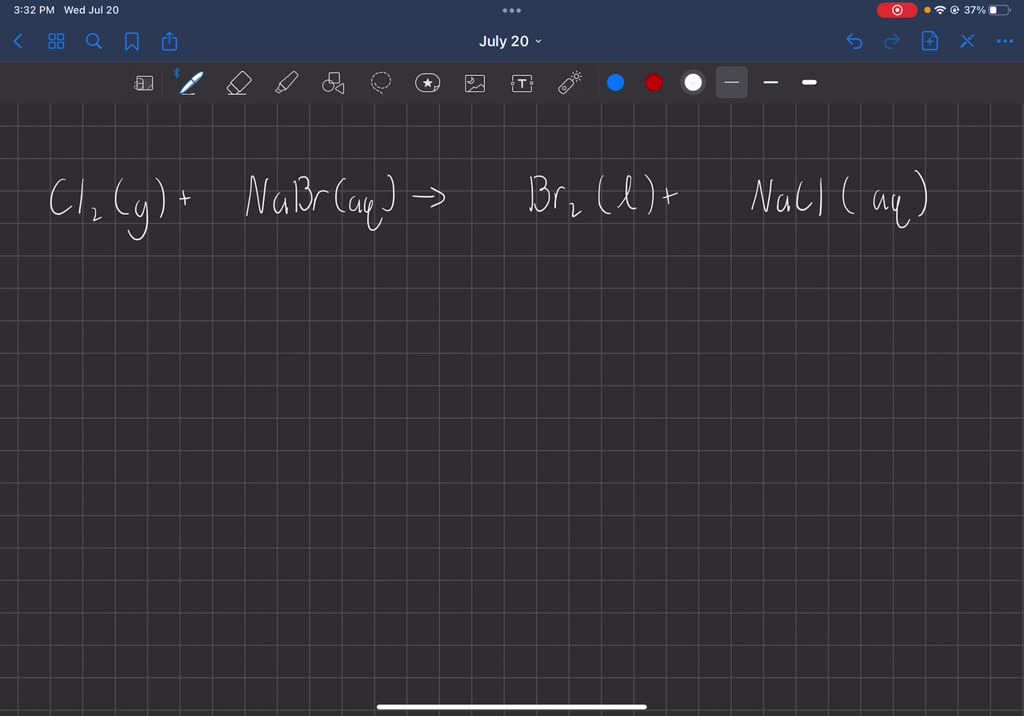
Bromine And Chlorine Gas Equation . cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous.
from www.numerade.com
nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide.
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are
Bromine And Chlorine Gas Equation The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous.
From brainly.com
Chlorine gas reacts with aqueous sodium bromide to produce aqueous Bromine And Chlorine Gas Equation albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is. Bromine And Chlorine Gas Equation.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine And Chlorine Gas Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the reaction between c=c double bond and bromine (br 2) can be used as a test for the. Bromine And Chlorine Gas Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Bromine And Chlorine Gas Equation The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. The reaction between chlorine. Bromine And Chlorine Gas Equation.
From pediaa.com
Difference Between Bromine and Chlorine Bromine And Chlorine Gas Equation the relative lower reactivity of bromine makes it exhibits a much greater selectivity. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between chlorine gas and. Bromine And Chlorine Gas Equation.
From www.youtube.com
Balancing and Writing the Equation for Sodium + Chlorine gas YouTube Bromine And Chlorine Gas Equation The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Bromine And Chlorine Gas Equation revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. albr3 + cl = alcl3 + br. Bromine And Chlorine Gas Equation.
From www.nagwa.com
Question Video Identifying the Product of the Reaction of Ethyne Gas Bromine And Chlorine Gas Equation The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. cl 2 (aq) + 2ki. Bromine And Chlorine Gas Equation.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. nabr + cl = br +. Bromine And Chlorine Gas Equation.
From fyoidypaj.blob.core.windows.net
Bromine Sodium Hydroxide Equation at Robert Herron blog Bromine And Chlorine Gas Equation The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. revision notes on. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce Bromine And Chlorine Gas Equation when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution. Bromine And Chlorine Gas Equation.
From fyorzqilq.blob.core.windows.net
Bromine And Chlorine Ionic Equation at Kelly McFadden blog Bromine And Chlorine Gas Equation nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. The reaction. Bromine And Chlorine Gas Equation.
From www.chegg.com
Solved Part A Write a balanced equation for the reaction of Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Potassium bromide + chlorine = potassium chloride + bromine Bromine And Chlorine Gas Equation the relative lower reactivity of bromine makes it exhibits a much greater selectivity. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The bromine. Bromine And Chlorine Gas Equation.
From www.yakimankagbu.ru
What Is The Equation For The Reaction With Chlorine And, 45 OFF Bromine And Chlorine Gas Equation The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. nabr + cl =. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVEDGaseous chlorine will displace bromide ion from an aqueous Bromine And Chlorine Gas Equation The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. the relative lower reactivity of. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED What volume of bromine is required to react with 145 liters of Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. the relative lower reactivity of bromine makes it exhibits a much greater. Bromine And Chlorine Gas Equation.
From www.youtube.com
How to Balance NaBr + Cl2 = NaCl + Br2 (Sodium Bromide + Chlorine gas Bromine And Chlorine Gas Equation revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Solid aluminum metal and diatomic bromine liquid react Bromine And Chlorine Gas Equation The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. revision notes on 2.3.2 halogen displacement reactions for the edexcel a. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for each reaction. a. Solid Bromine And Chlorine Gas Equation nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. albr3 + cl =. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED For the following reaction, 6.37 grams of chlorine gas are Bromine And Chlorine Gas Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the reaction between c=c double bond and bromine (br 2) can be used as a test for the. Bromine And Chlorine Gas Equation.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. the relative lower reactivity of bromine. Bromine And Chlorine Gas Equation.
From exylzaqxv.blob.core.windows.net
Bromine And Chlorine Together at Angela Tucker blog Bromine And Chlorine Gas Equation The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample.. Bromine And Chlorine Gas Equation.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Bromine And Chlorine Gas Equation the relative lower reactivity of bromine makes it exhibits a much greater selectivity. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. cl 2 (aq) + 2ki (aq) → 2kcl (aq) +. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED According to the following reaction, how many grams of chlorine Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level. Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Metals bond with halogens to form colorless metal halides Bromine And Chlorine Gas Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. The reaction between. Bromine And Chlorine Gas Equation.
From brainly.com
Aluminum bromide and chlorine gas react to form aluminum chloride and Bromine And Chlorine Gas Equation the reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. when chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. . Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to Bromine And Chlorine Gas Equation albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. The bromine reagent. Bromine And Chlorine Gas Equation.
From www.numerade.com
When chlorine gas is bubbled into a solution of sodium bromide, the Bromine And Chlorine Gas Equation The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. the reaction. Bromine And Chlorine Gas Equation.
From studylib.net
Displacement reactions Reactions of chlorine Bromine And Chlorine Gas Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. The bromine. Bromine And Chlorine Gas Equation.
From exyanngrz.blob.core.windows.net
Chlorine And Bromine Ionic Compound at Jason Hamlett blog Bromine And Chlorine Gas Equation The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. the relative. Bromine And Chlorine Gas Equation.
From www.chegg.com
Solved P1 The law for the hydrogenbromine reaction Bromine And Chlorine Gas Equation revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. The reaction between chlorine gas and sodium bromide produces liquid bromine and aqueous. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. The bromine reagent is in reddish color, and the product vicinal dibromide is colorless. . Bromine And Chlorine Gas Equation.
From www.numerade.com
SOLVED 38. Write balanced chemical equation for the following reaction Bromine And Chlorine Gas Equation albr3 + cl = alcl3 + br is a single displacement (substitution) reaction where one mole of aqueous aluminum bromide. nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns. Bromine And Chlorine Gas Equation.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine And Chlorine Gas Equation cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine solution forms. the relative lower reactivity of bromine makes it exhibits a much greater selectivity. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. the reaction between c=c. Bromine And Chlorine Gas Equation.
From www.coursehero.com
[Solved] The reaction between bromine and ethane occurs in the presence Bromine And Chlorine Gas Equation nabr + cl = br + nacl is a single displacement (substitution) reaction where one mole of aqueous sodium bromide. revision notes on 2.3.2 halogen displacement reactions for the edexcel a level chemistry syllabus, written by the. cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the reaction mixture turns darker and iodine. Bromine And Chlorine Gas Equation.