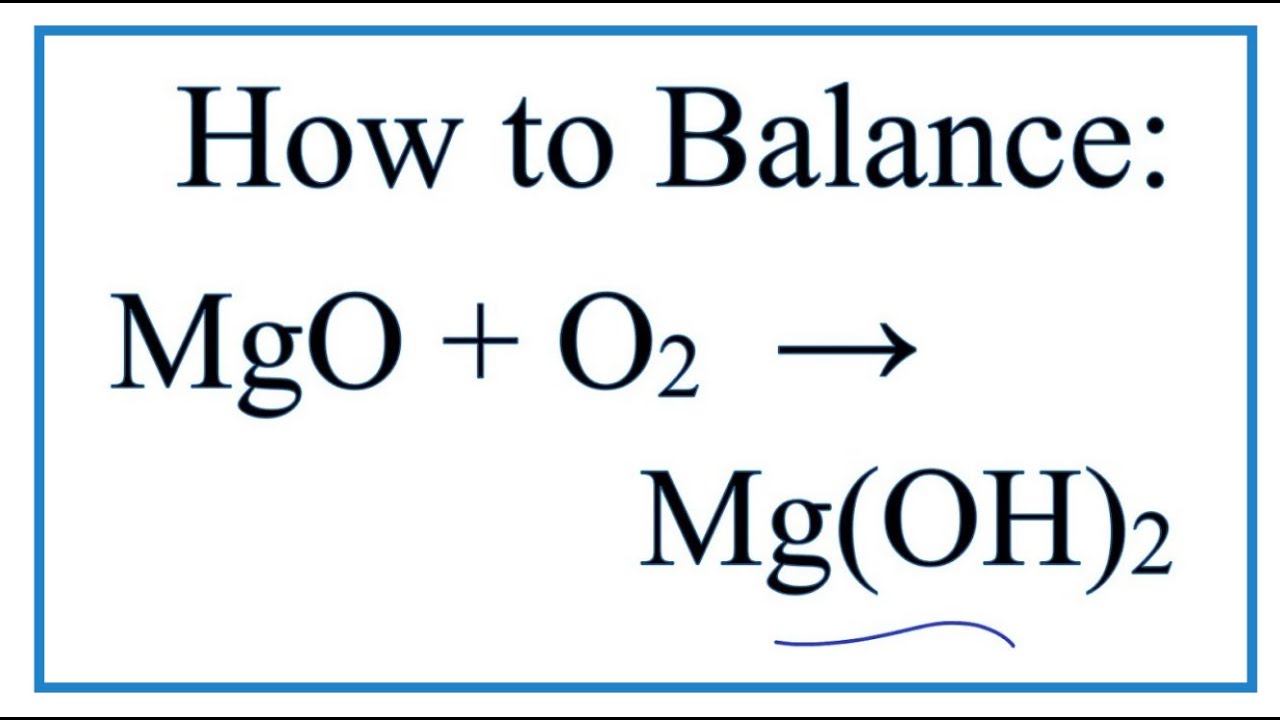Magnesium Oxide Reacts With Water . magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. by burning a strip of magnesium, we obtain magnesium oxide which we then. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white magnesium oxide and hydrogen gas.
from www.youtube.com
magnesium burns in steam to produce white magnesium oxide and hydrogen gas. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. by burning a strip of magnesium, we obtain magnesium oxide which we then. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}.
How to Balance MgO + H2O = Mg(OH)2 (Magnesium Oxide plus Water) YouTube
Magnesium Oxide Reacts With Water for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. by burning a strip of magnesium, we obtain magnesium oxide which we then.
From childhealthpolicy.vumc.org
Chemical equation for magnesium oxide. Magnesium oxide lab. 20221010 Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. what happens with the magnesium is that a small initial reaction coats. Magnesium Oxide Reacts With Water.
From www.coursehero.com
[Solved] Magnesium oxide reacts with phosphoric acid to produce Magnesium Oxide Reacts With Water by burning a strip of magnesium, we obtain magnesium oxide which we then. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. magnesium. Magnesium Oxide Reacts With Water.
From kelasmenggambarbagus866.blogspot.com
Solid Magnesium Hydroxide Reacts With Hydrochloric Acid Solved Magnesium Oxide Reacts With Water by burning a strip of magnesium, we obtain magnesium oxide which we then. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. . Magnesium Oxide Reacts With Water.
From thefitnessmanual.com
Magnesium Reacts With Cold Water TheFitnessManual Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. by burning a strip of magnesium, we obtain magnesium oxide which we then. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. revision notes on 6.1.4 oxides reacting with water for. Magnesium Oxide Reacts With Water.
From www.sciencephoto.com
Magnesium reacts with water Stock Image C028/0749 Science Photo Magnesium Oxide Reacts With Water for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. by burning. Magnesium Oxide Reacts With Water.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Magnesium Oxide Reacts With Water what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. \[ mg_{(s)}. Magnesium Oxide Reacts With Water.
From analiticaderetail.com
Csillogás Ugró jack Látható magnesium oxide hydrogen reaction Ananiver Magnesium Oxide Reacts With Water for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. revision notes on. Magnesium Oxide Reacts With Water.
From www.youtube.com
Magnesium Oxide Reaction With Water YouTube Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. by burning a strip of magnesium, we obtain magnesium oxide which we then. magnesium burns in steam to. Magnesium Oxide Reacts With Water.
From mammothmemory.net
Magnesium introduced to steam produces a bright flame Magnesium Oxide Reacts With Water by burning a strip of magnesium, we obtain magnesium oxide which we then. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce. Magnesium Oxide Reacts With Water.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to.. Magnesium Oxide Reacts With Water.
From www.numerade.com
Magnesium carbonate into magnesium Magnesium Oxide Reacts With Water \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce. Magnesium Oxide Reacts With Water.
From www.sciencephoto.com
Magnesium reacting with water Stock Image A500/0369 Science Photo Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. magnesium burns in steam to produce white. Magnesium Oxide Reacts With Water.
From askfilo.com
Magnesium reacts with oxygen to form magnesium oxide. This is an example Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry. Magnesium Oxide Reacts With Water.
From www.numerade.com
SOLVED Text Magnesium oxide reacts with hydrochloric acid and Magnesium Oxide Reacts With Water \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry. Magnesium Oxide Reacts With Water.
From www.youtube.com
How to Balance MgO + H2O = Mg(OH)2 (Magnesium Oxide plus Water) YouTube Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in. Magnesium Oxide Reacts With Water.
From gioqrodfr.blob.core.windows.net
Magnesium Water Oxide at Holly Kawamura blog Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble.. Magnesium Oxide Reacts With Water.
From www.sciencephoto.com
Magnesium oxide reacts with water Stock Image C030/8190 Science Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. what happens with the. Magnesium Oxide Reacts With Water.
From www.nagwa.com
Question Video Identifying the Name of the Gas Produced When Magnesium Magnesium Oxide Reacts With Water for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. by burning a strip of magnesium, we obtain magnesium oxide which we then. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. what happens with the magnesium is that a small initial reaction coats the. Magnesium Oxide Reacts With Water.
From www.quora.com
Why do magnesium and calcium not react with water, while sodium and Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. magnesium burns in steam. Magnesium Oxide Reacts With Water.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium Oxide Reacts With Water for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. what happens with the magnesium is. Magnesium Oxide Reacts With Water.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. by burning a strip of magnesium, we obtain magnesium oxide which we then. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. revision notes on 6.1.4 oxides. Magnesium Oxide Reacts With Water.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Oxide Reacts With Water \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean.. Magnesium Oxide Reacts With Water.
From pixels.com
Magnesium Reacting With Acid Photograph by Andrew Lambert Photography Magnesium Oxide Reacts With Water by burning a strip of magnesium, we obtain magnesium oxide which we then. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. revision notes on 6.1.4 oxides reacting with water for. Magnesium Oxide Reacts With Water.
From brainly.in
What happens when Magnesium oxide is dissolved in water? Write a word Magnesium Oxide Reacts With Water for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. by burning a strip of magnesium, we obtain magnesium oxide which we then. what happens with. Magnesium Oxide Reacts With Water.
From www.meritnation.com
why calcium and magnesium floats on water Science Metals and Non Magnesium Oxide Reacts With Water what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white. Magnesium Oxide Reacts With Water.
From www.youtube.com
The reaction between Magnesium oxide (MgO) & Water (H2O) MgO+H2O YouTube Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. revision notes on 6.1.4 oxides reacting with water for the aqa a. Magnesium Oxide Reacts With Water.
From mammothmemory.net
Magnesium reacts with water but not vigorously Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will. Magnesium Oxide Reacts With Water.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium Oxide Reacts With Water what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. by. Magnesium Oxide Reacts With Water.
From www.youtube.com
Reaction of Magnesium and Water (Mg + H2O) YouTube Magnesium Oxide Reacts With Water what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}.. Magnesium Oxide Reacts With Water.
From fphoto.photoshelter.com
science chemistry redox reaction magnesium hydrochloric acid Magnesium Oxide Reacts With Water Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. by burning a strip of magnesium,. Magnesium Oxide Reacts With Water.
From www.numerade.com
SOLVED Give the formula for the compound formed when sulfur dioxide Magnesium Oxide Reacts With Water by burning a strip of magnesium, we obtain magnesium oxide which we then. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. magnesium. Magnesium Oxide Reacts With Water.
From astonishingceiyrs.blogspot.com
Sodium Oxide Reacts With Water astonishingceiyrs Magnesium Oxide Reacts With Water revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer. Magnesium Oxide Reacts With Water.
From askfilo.com
calcium oxide reacts with water, calcium hydroxide is formed. magnesium r.. Magnesium Oxide Reacts With Water magnesium burns in steam to produce white magnesium oxide and hydrogen gas. \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. for starters combusted magnesium is going to produce partially amorphous $\ce{mgo}$ which will reduce the activation energy to. by burning a strip of magnesium, we obtain magnesium oxide which we then. magnesium burns in steam to. Magnesium Oxide Reacts With Water.
From www.sciencephoto.com
Magnesium oxide reacts with water Stock Image C030/8188 Science Magnesium Oxide Reacts With Water \[ mg_{(s)} + h_2o_{(g)} \rightarrow mgo_{(s)} + h_{2(g)}. by burning a strip of magnesium, we obtain magnesium oxide which we then. magnesium burns in steam to produce white magnesium oxide and hydrogen gas. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. magnesium burns in steam to produce white magnesium oxide. Magnesium Oxide Reacts With Water.
From www.numerade.com
SOLVED 16 For the reaction MgOts) HzOv) Mg(OH)2(s) 4H 37.7 kJ Magnesium Oxide Reacts With Water what happens with the magnesium is that a small initial reaction coats the surface of it with a very thin layer of insoluble. Mg (s) + h 2 o (g) mgo (s) + h 2 (g) very clean. by burning a strip of magnesium, we obtain magnesium oxide which we then. magnesium burns in steam to produce. Magnesium Oxide Reacts With Water.