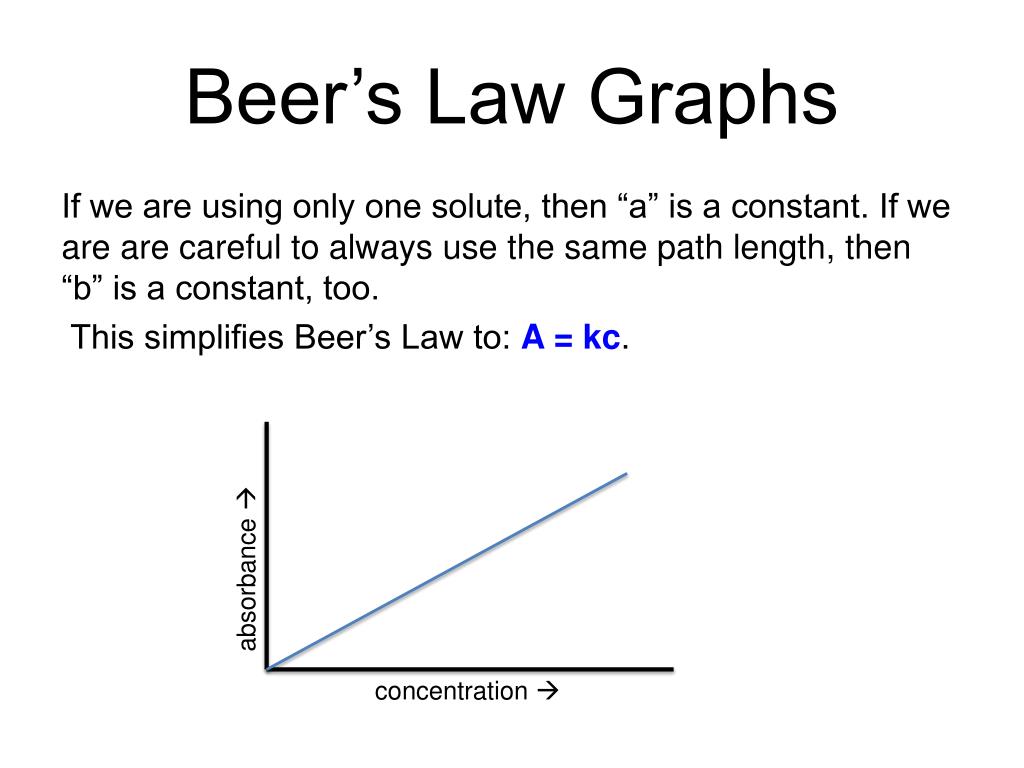
What Does Beer's Law Mean . in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. It is also referred to as beer’s law.
from www.slideserve.com
It is also referred to as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and.
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download
What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. It is also referred to as beer’s law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law What Does Beer's Law Mean It is also referred to as beer’s law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. “the path length and concentration. What Does Beer's Law Mean.
From www.geeksforgeeks.org
BeerLambert Law Statement, Formula, Equation & Derivation What Does Beer's Law Mean “the path length and concentration of a chemical are directly proportional to its absorption of light.”. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,.. What Does Beer's Law Mean.
From sciencenotes.org
Beer's Law Equation and Example What Does Beer's Law Mean since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. It is also referred to as beer’s law. in spectroscopy, beer’s law states that the. What Does Beer's Law Mean.
From lelandchemclub.weebly.com
Solutions Leland Chemistry Club What Does Beer's Law Mean beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. . What Does Beer's Law Mean.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Beer's Law Mean beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. “the path length and concentration of a chemical are directly proportional to its. What Does Beer's Law Mean.
From chemistrysources.com
قانون بيير (بير) Beer's Law التعريف و المعادلة مصادر الكيمياء What Does Beer's Law Mean in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an. What Does Beer's Law Mean.
From www.youtube.com
Beer's Law Overview YouTube What Does Beer's Law Mean in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of. What Does Beer's Law Mean.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Beer's Law Mean “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. It is also referred to as beer’s law. beer’s law states that the light absorbance of a solution is directly proportional to the. What Does Beer's Law Mean.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Beer's Law Mean It is also referred to as beer’s law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path. What Does Beer's Law Mean.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Beer's Law Mean “the path length and concentration of a chemical are directly proportional to its absorption of light.”. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write. What Does Beer's Law Mean.
From studylib.net
Beer's Law What Does Beer's Law Mean It is also referred to as beer’s law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law, in spectroscopy, a relation. What Does Beer's Law Mean.
From fyowvisuc.blob.core.windows.net
Beer's Law Absorbance Units at Patrick Glisson blog What Does Beer's Law Mean It is also referred to as beer’s law. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. “the path length and concentration of a chemical are directly. What Does Beer's Law Mean.
From pdfprof.com
La loi de Beer Lambert LHCE What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. It is also referred to as beer’s law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. “the path length and concentration of a chemical are directly. What Does Beer's Law Mean.
From www.slideserve.com
PPT Spectrophotometry PowerPoint Presentation, free download ID795528 What Does Beer's Law Mean It is also referred to as beer’s law. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path. What Does Beer's Law Mean.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. It is. What Does Beer's Law Mean.
From www.slideserve.com
PPT Beer’s Law and Spectrophotometry PowerPoint Presentation ID211563 What Does Beer's Law Mean in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. It is also referred to as beer’s law. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law states that the light. What Does Beer's Law Mean.
From www.adda247.com
Beer Lambert Law Equation Derivation, Formula, Examples What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an. What Does Beer's Law Mean.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 What Does Beer's Law Mean since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. It is also referred to. What Does Beer's Law Mean.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. It. What Does Beer's Law Mean.
From www.geeksforgeeks.org
Difference Between Beer’s Law and Lambert’s Law What Does Beer's Law Mean in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. It is also referred to as beer’s law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. “the path length and concentration. What Does Beer's Law Mean.
From www.thoughtco.com
Beer's Law Definition and Equation What Does Beer's Law Mean since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. It is also referred to as beer’s law. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the. What Does Beer's Law Mean.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of. What Does Beer's Law Mean.
From studylib.net
Beer`s Law What Does Beer's Law Mean beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly. What Does Beer's Law Mean.
From www.slideserve.com
PPT Spectrochemical Analysis PowerPoint Presentation ID478352 What Does Beer's Law Mean It is also referred to as beer’s law. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing. What Does Beer's Law Mean.
From www.slideserve.com
PPT Review of Basic Concepts, Molarity, Solutions, Dilutions and Beer What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. beer’s. What Does Beer's Law Mean.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News What Does Beer's Law Mean since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. “the path length and concentration of a chemical are directly proportional to its absorption of light.”.. What Does Beer's Law Mean.
From facts.net
13 Surprising Facts About BeerLambert Law What Does Beer's Law Mean It is also referred to as beer’s law. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. Beer lamberts law states a relationship between the attenuation of light through a substance. What Does Beer's Law Mean.
From www.youtube.com
Beer's Law Concept, Calculations, & Lab YouTube What Does Beer's Law Mean “the path length and concentration of a chemical are directly proportional to its absorption of light.”. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. It is also referred to as beer’s law. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. What Does Beer's Law Mean.
From www.thoughtco.com
Beer's Law Definition and Equation What Does Beer's Law Mean beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. It is also referred to as beer’s law. “the path length and concentration of a chemical are directly proportional to its absorption of light.”. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. What Does Beer's Law Mean.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation, free download What Does Beer's Law Mean It is also referred to as beer’s law. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the absorption of. What Does Beer's Law Mean.
From gioeuwgjx.blob.core.windows.net
Beer's Law Equation Units at Virginia Haney blog What Does Beer's Law Mean beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy. What Does Beer's Law Mean.
From www.youtube.com
BeerLambert law in easy way YouTube What Does Beer's Law Mean beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can. What Does Beer's Law Mean.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download What Does Beer's Law Mean Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of that substance. beer’s law states that the light absorbance of a solution is directly proportional to the concentration of the attenuating species and. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. . What Does Beer's Law Mean.
From www.youtube.com
Ch 17 7 Beer's Law is Additive for All Components YouTube What Does Beer's Law Mean since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. “the path length and concentration of a chemical are directly proportional to its. What Does Beer's Law Mean.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download What Does Beer's Law Mean in spectroscopy, beer’s law states that the absorption of light by a sample is directly proportional to the length of its path and its. beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer lamberts law states a relationship between the attenuation of light through a substance and the properties of. What Does Beer's Law Mean.