Chlorine Atom In The Third Excited State . Full ground state electron configuration: An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited.
from www.elevise.co.uk
In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Full ground state electron configuration: The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common.
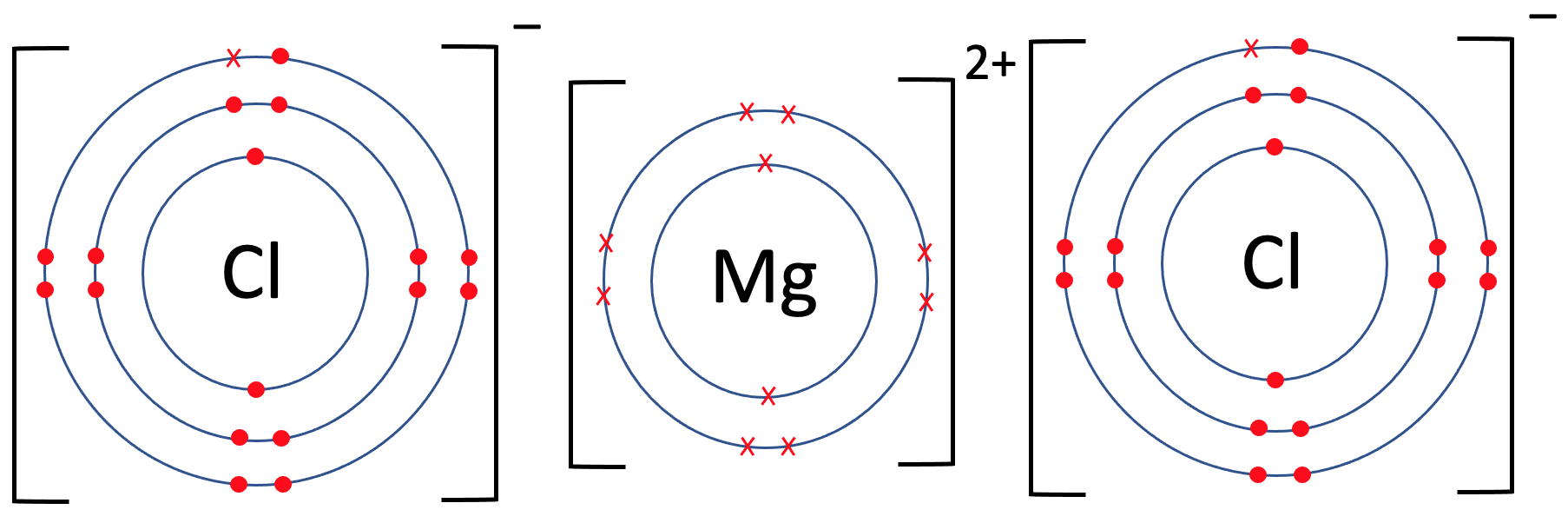
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise
Chlorine Atom In The Third Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Full ground state electron configuration: The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state.
From www.toppr.com
In which of the excitation state of chlorine CIF, is 4. formed (1) In Chlorine Atom In The Third Excited State The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Full ground state electron configuration: In order to write the chlorine electron configuration we first need to know the number of electrons for the. Chlorine Atom In The Third Excited State.
From www.numerade.com
SOLVEDA chlorine atom in its ground state has a total of seven Chlorine Atom In The Third Excited State Full ground state electron configuration: An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Because chlorine is so highly reactive, it is found in. Chlorine Atom In The Third Excited State.
From www.chemistrylearner.com
Chlorine Facts, Symbol, Discovery, Properties, Uses Chlorine Atom In The Third Excited State Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a. Chlorine Atom In The Third Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Chlorine Atom In The Third Excited State The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Full ground state electron configuration: Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. An excited state is an energy level of an atom, ion, or molecule in which. Chlorine Atom In The Third Excited State.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Chlorine Atom In The Third Excited State The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. In. Chlorine Atom In The Third Excited State.
From www.doubtnut.com
In the third excited state the number of unparied electrons in chlorin Chlorine Atom In The Third Excited State In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. The arrangement of electrons in the orbitals of an atom is called. Chlorine Atom In The Third Excited State.
From animalia-life.club
Cl2 Molecular Geometry Chlorine Atom In The Third Excited State Full ground state electron configuration: To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. An excited state is an energy level of an atom,. Chlorine Atom In The Third Excited State.
From www.researchgate.net
Structures obtained for the ion pairs of chloroethane, at the Chlorine Atom In The Third Excited State In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Full ground state electron configuration: Because chlorine is. Chlorine Atom In The Third Excited State.
From www.toppr.com
58. Degeneracy of Hatom in 3rd excited state is (1) 16 (2) 10 (3) 12 Chlorine Atom In The Third Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17. Chlorine Atom In The Third Excited State.
From www.slideserve.com
PPT Ground vs. Excited State PowerPoint Presentation, free download Chlorine Atom In The Third Excited State Full ground state electron configuration: To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The arrangement of electrons in the orbitals. Chlorine Atom In The Third Excited State.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Atom In The Third Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The arrangement of electrons in the orbitals of an atom. Chlorine Atom In The Third Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Full ground state electron configuration: An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Because chlorine is so highly reactive, it is found in. Chlorine Atom In The Third Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Chlorine Atom In The Third Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. To consider what happens in the process of fluorescence, we need. Chlorine Atom In The Third Excited State.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine Atom In The Third Excited State In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Because chlorine is so highly reactive, it is found in nature in a combined state with other. Chlorine Atom In The Third Excited State.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Atom In The Third Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. In order to write the chlorine electron configuration we first need to know the number. Chlorine Atom In The Third Excited State.
From rwu.pressbooks.pub
Chapter 12. Photosynthesis Introduction to Molecular and Cell Biology Chlorine Atom In The Third Excited State Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Full ground state electron configuration: In order to write the chlorine. Chlorine Atom In The Third Excited State.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chlorine Atom In The Third Excited State Full ground state electron configuration: Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. An excited state is an energy level of an atom, ion, or molecule in which. Chlorine Atom In The Third Excited State.
From www.vrogue.co
Excited State Atom Chemistry Libretexts vrogue.co Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The arrangement of electrons in the orbitals of an atom is called the electron configuration of the. Chlorine Atom In The Third Excited State.
From brainly.in
Draw the electron dot structure of chlorine molecule Brainly.in Chlorine Atom In The Third Excited State Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The arrangement of electrons in the orbitals of an atom is called the. Chlorine Atom In The Third Excited State.
From www.chegg.com
Solved A hydrogen atom is in its third excited state (n = Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In order to write the chlorine electron configuration we first need to know the number. Chlorine Atom In The Third Excited State.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). An excited state is an energy level of an atom, ion, or molecule in which an electron. Chlorine Atom In The Third Excited State.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Chlorine Atom In The Third Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. Full ground state electron configuration: The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Because chlorine is so highly reactive, it is found in nature in a combined. Chlorine Atom In The Third Excited State.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Chlorine Atom In The Third Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The arrangement of electrons in the orbitals of an atom is called. Chlorine Atom In The Third Excited State.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). An excited state is an energy level of an atom, ion, or molecule in which an electron. Chlorine Atom In The Third Excited State.
From saylordotorg.github.io
Ions Chlorine Atom In The Third Excited State Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Full ground. Chlorine Atom In The Third Excited State.
From www.toppr.com
Chlorine atom in its third excited state with fluorine to form a Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. In. Chlorine Atom In The Third Excited State.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Atom In The Third Excited State An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Because chlorine is so highly reactive, it is. Chlorine Atom In The Third Excited State.
From www.doubtnut.com
Chlorine atom in the third excited state reacts with Fluorine to form Chlorine Atom In The Third Excited State Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a. Chlorine Atom In The Third Excited State.
From pixels.com
Chlorine Electron Configuration Photograph by Chlorine Atom In The Third Excited State The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a. Chlorine Atom In The Third Excited State.
From chlorine-atom.weebly.com
Structure Chlorine Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. An. Chlorine Atom In The Third Excited State.
From www.numerade.com
SOLVED What change is occurring in this figure? Nat Sodium atom Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. Because chlorine is so highly reactive, it is found in nature in a combined state. Chlorine Atom In The Third Excited State.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Atom In The Third Excited State In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Full ground state electron configuration: The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Because chlorine is so highly reactive, it is found in nature in. Chlorine Atom In The Third Excited State.
From www.pinterest.com
Excited State Easy Science Easy science, Electron configuration, Ap Chlorine Atom In The Third Excited State To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. An excited state is an energy level of an atom, ion, or molecule in which an electron is at a higher energy level than its ground state. The arrangement of electrons in the orbitals of an atom. Chlorine Atom In The Third Excited State.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Atom In The Third Excited State Full ground state electron configuration: To consider what happens in the process of fluorescence, we need to think of the possible energy states for a ground and excited. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. In order to write the chlorine electron configuration we first. Chlorine Atom In The Third Excited State.
From byjus.com
ntMaximum value (n+l+m) for unpaired electrons in second excited state Chlorine Atom In The Third Excited State The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Because chlorine is so highly reactive, it is found in nature in a combined state with other elements, such as nacl (common. Full ground state electron configuration: The arrangement of electrons in the orbitals of an atom is called the electron configuration. Chlorine Atom In The Third Excited State.