Medical Device Risk Management Fda . Identify when to use risk management. This tir provides guidance for addressing postmarket security risk management within the risk management. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Discuss the reasons for conducting risk management activities for medical devices. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward.
from www.complianceonline.com
2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. This tir provides guidance for addressing postmarket security risk management within the risk management. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk management. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the.
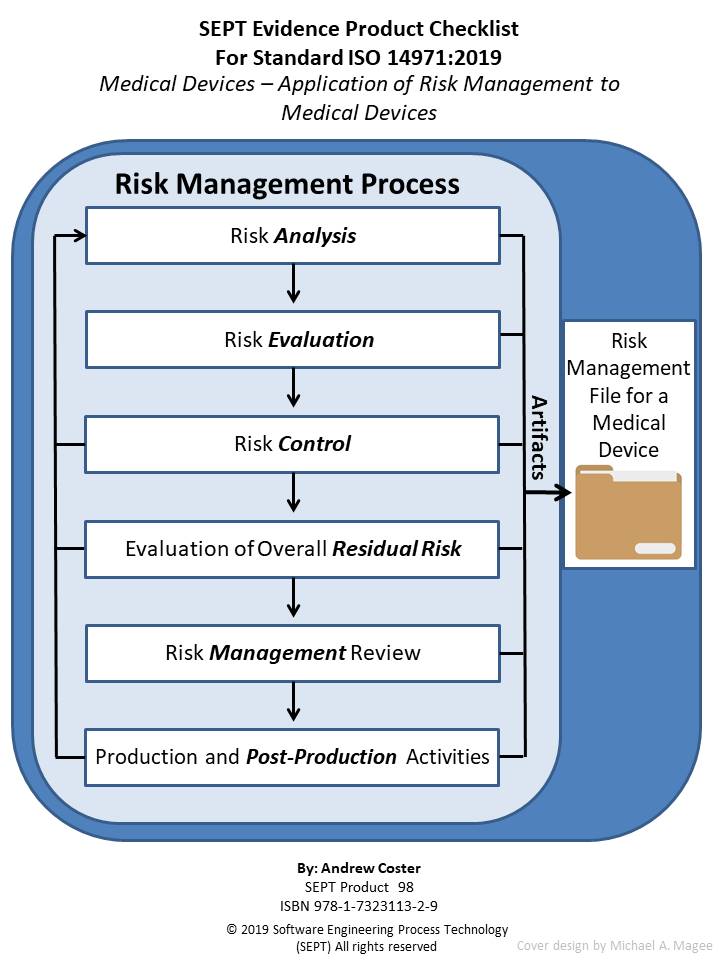
ISO 149712019 Medical devices Application of Risk Management to
Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. This tir provides guidance for addressing postmarket security risk management within the risk management. Identify when to use risk management. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. Discuss the reasons for conducting risk management activities for medical devices. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that.
From kvalito.ch
Risk Management for Medical Devices ISO 149712019 Kvalito Medical Device Risk Management Fda The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. This tir provides guidance for addressing postmarket security risk management within the risk management. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. •. Medical Device Risk Management Fda.
From www.meddeviceonline.com
Navigating The Universe Of Risk In Medical Device Development Medical Device Risk Management Fda Identify when to use risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape. Medical Device Risk Management Fda.
From www.pinterest.com
How the US FDA classifies Medical Devices Risk management, Regulatory Medical Device Risk Management Fda Identify when to use risk management. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. A common understanding of how fda considers benefit and. Medical Device Risk Management Fda.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Risk Management Fda Discuss the reasons for conducting risk management activities for medical devices. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. 2, 2024, the fda. Medical Device Risk Management Fda.
From medicaldevicehq.com
Infographic Risk management for medical device and ISO 149712019 Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. • risk, risk analysis and risk management spans the full total product lifecycle of medical. Medical Device Risk Management Fda.
From medicaldevicehq.com
Usability engineering and ISO 14971 risk management for medical devices Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by. Medical Device Risk Management Fda.
From www.presentationeze.com
Medical Device Risk PresentationEZE Medical Device Risk Management Fda The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. A common understanding of how fda considers benefit and risk may better align industry’s and. Medical Device Risk Management Fda.
From www.orielstat.com
ISO 149712019 Basics of Medical Device Risk Management Medical Device Risk Management Fda The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. This tir provides guidance for addressing postmarket security risk management within the risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. Identify when to use risk management.. Medical Device Risk Management Fda.
From qualcy.com
Medical Device Quality Risk Management · Qualcy eQMS Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. This tir provides guidance for addressing postmarket security risk management within the risk management. Identify. Medical Device Risk Management Fda.
From spyro-soft.com
ISO 14971 Risk Management for Medical Devices explained Medical Device Risk Management Fda This tir provides guidance for addressing postmarket security risk management within the risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. Discuss the reasons for conducting risk management activities for medical devices. • risk, risk analysis and risk management spans the full total product lifecycle of medical. Medical Device Risk Management Fda.
From array.aami.org
Documenting Medical Device Risk Management through the Risk Medical Device Risk Management Fda The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. This tir provides guidance for addressing postmarket security risk management within the risk management. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. 2,. Medical Device Risk Management Fda.
From www.qualitydigest.com
Understanding ISO 14971 Medical Device Risk Management Quality Digest Medical Device Risk Management Fda Discuss the reasons for conducting risk management activities for medical devices. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. • risk, risk analysis and risk management spans the. Medical Device Risk Management Fda.
From www.orielstat.com
Choosing the right medical device risk management tools Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. This tir provides guidance for addressing postmarket security risk management within the risk management. Discuss the reasons for conducting risk management activities for medical devices. • risk, risk analysis and risk management spans the full. Medical Device Risk Management Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Risk Management Fda The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. Identify when to use risk management. • risk, risk analysis and risk management spans the full total product lifecycle of. Medical Device Risk Management Fda.
From www.greenlight.guru
ISO 14971 Risk Management for Medical Devices [Guide] Medical Device Risk Management Fda Discuss the reasons for conducting risk management activities for medical devices. This tir provides guidance for addressing postmarket security risk management within the risk management. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. A common understanding of how fda considers benefit and risk may better align industry’s and. Medical Device Risk Management Fda.
From www.slideserve.com
PPT Medical Device Risk Management Practical Overview & Challenges Medical Device Risk Management Fda This tir provides guidance for addressing postmarket security risk management within the risk management. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. Discuss the reasons for conducting risk management activities for medical devices. The process described in this document intends to assist manufacturers. Medical Device Risk Management Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on. Medical Device Risk Management Fda.
From www.presentationeze.com
FDA medical device classification PresentationEZE Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. This tir provides guidance for addressing postmarket security risk management. Medical Device Risk Management Fda.
From medicaldevicehq.com
How to integrate proactive safety by design with medical device risk Medical Device Risk Management Fda Discuss the reasons for conducting risk management activities for medical devices. This tir provides guidance for addressing postmarket security risk management within the risk management. Identify when to use risk management. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. A common understanding of. Medical Device Risk Management Fda.
From www.slideshare.net
Effective Risk Management For Medical Devices inar Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. This tir provides guidance for addressing postmarket security risk management within the risk management. Identify when to use risk management. Discuss the reasons for conducting risk management activities for medical devices. 2, 2024, the fda. Medical Device Risk Management Fda.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by. Medical Device Risk Management Fda.
From www.reedtech.com
Medical Device Risk Management FMEA Medical Devices Reed Tech Medical Device Risk Management Fda Identify when to use risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. Discuss the reasons for conducting risk management activities for. Medical Device Risk Management Fda.
From www.vrogue.co
Risk Management For Medical Device And Iso 149712019 vrogue.co Medical Device Risk Management Fda Discuss the reasons for conducting risk management activities for medical devices. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Identify when to use. Medical Device Risk Management Fda.
From medicaldeviceacademy.com
Overview of Similarities and Differences between QSR and ISO 13485 Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Discuss the reasons for conducting risk management activities for medical devices. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a. Medical Device Risk Management Fda.
From www.greenlight.guru
Ultimate Guide to ISO 13485 Quality Management System (QMS) for Medical Medical Device Risk Management Fda The process described in this document intends to assist manufacturers of medical devices to identify the hazards associated with the. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. This tir provides guidance for addressing postmarket security risk management within the risk management. •. Medical Device Risk Management Fda.
From arithmostech.com
Risk management requirements for PMS for medical devices Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Discuss the reasons for conducting risk management activities for medical devices. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a. Medical Device Risk Management Fda.
From corsi-dm.it
Introduction to Medical Device Safety Risk Management in Compliance Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. This tir provides guidance for addressing postmarket security risk management within the risk management. The. Medical Device Risk Management Fda.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. Discuss the reasons for conducting risk management activities for medical devices. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. Identify when to use. Medical Device Risk Management Fda.
From www.greenlight.guru
SaMD Software as a Medical Device [The Ultimate Guide] Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. Discuss the reasons for conducting risk management activities for medical devices. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. Identify when to use. Medical Device Risk Management Fda.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Risk Management Fda Discuss the reasons for conducting risk management activities for medical devices. Identify when to use risk management. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on. Medical Device Risk Management Fda.
From exeedqm.com
FDA Guidance Shows a Regulatory Path Forward for Interoperable Devices Medical Device Risk Management Fda A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. Identify when to use risk management. This tir provides guidance for addressing postmarket security risk management within the risk management. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management. Medical Device Risk Management Fda.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. This tir provides guidance for addressing postmarket security risk management within the risk management. •. Medical Device Risk Management Fda.
From www.orielstat.com
Medical Device QMS 101 What It Is, Where It’s Required, and Key Medical Device Risk Management Fda This tir provides guidance for addressing postmarket security risk management within the risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. Discuss. Medical Device Risk Management Fda.
From www.complianceonline.com
ISO 149712019 Medical devices Application of Risk Management to Medical Device Risk Management Fda • risk, risk analysis and risk management spans the full total product lifecycle of medical devices • concepts of risk are included in fda regulations. 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. Identify when to use risk management. A common understanding of. Medical Device Risk Management Fda.
From aaos.org
An Overview of the FDA Approval Process for Devices Medical Device Risk Management Fda 2, 2024, the fda set a new cornerstone in the medical device regulatory landscape by releasing the new quality management system regulation (qmsr), a forward. This tir provides guidance for addressing postmarket security risk management within the risk management. A common understanding of how fda considers benefit and risk may better align industry’s and fda’s focus on actions that. •. Medical Device Risk Management Fda.