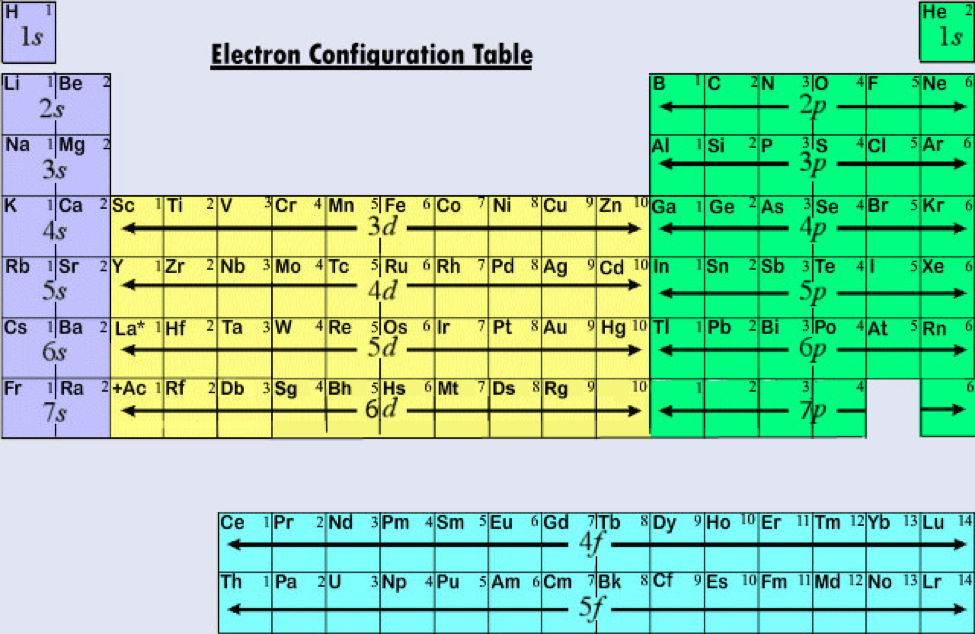
Rules Distribution Of Electrons . general rules for assigning electrons to atomic orbitals. Electron configurations of atoms follow a standard notation. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. All of the electrons in the noble gas neon (atomic number 10). fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Although the distributions of electrons. the most common way to describe electron configurations is to write distributions in the spdf notation. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. An atom consists of subatomic.
from alevelchemistry.co.uk
An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. general rules for assigning electrons to atomic orbitals. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. An atom consists of subatomic. All of the electrons in the noble gas neon (atomic number 10). Although the distributions of electrons. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. the electron configuration of an element describes how electrons are distributed in its atomic orbitals.
Electron Configurations Orbitals, Energy Levels and Ionisation Energy
Rules Distribution Of Electrons Electron configurations of atoms follow a standard notation. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. All of the electrons in the noble gas neon (atomic number 10). Electron configurations of atoms follow a standard notation. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. general rules for assigning electrons to atomic orbitals. Although the distributions of electrons. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. An atom consists of subatomic. the most common way to describe electron configurations is to write distributions in the spdf notation.
From www.studypool.com
SOLUTION Electronic configuration and rules for distribution of Rules Distribution Of Electrons general rules for assigning electrons to atomic orbitals. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. Although the distributions of electrons. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. An atom consists of subatomic. All of the electrons in the. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Distribution of electrons in various shells. PowerPoint Rules Distribution Of Electrons Although the distributions of electrons. An atom consists of subatomic. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. the most common way to describe electron configurations is to write distributions in the spdf notation. the distribution of electrons in shells of the atomic orbitals of an atom is known as its. Rules Distribution Of Electrons.
From www.youtube.com
Distribution of electron in K L M N Shell II Bohr & bury rule Il Rules Distribution Of Electrons An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. All of the electrons in the noble gas neon (atomic number 10). the most common way to describe electron configurations is to write distributions. Rules Distribution Of Electrons.
From slideplayer.com
Chapter 2 Chemistry Review. ppt download Rules Distribution Of Electrons general rules for assigning electrons to atomic orbitals. An atom consists of subatomic. Electron configurations of atoms follow a standard notation. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. all we need to know is how many protons it has (and how many electrons, which is. Rules Distribution Of Electrons.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Rules Distribution Of Electrons An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. Although the distributions of electrons. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation. An atom consists of subatomic. All of the electrons in the noble gas neon (atomic number. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Structure of Atom PowerPoint Presentation, free download ID6503501 Rules Distribution Of Electrons fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. All of the electrons in the noble gas neon (atomic number 10). An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. An atom. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Electron Configurations PowerPoint Presentation, free download Rules Distribution Of Electrons the electron configuration of an element describes how electrons are distributed in its atomic orbitals. All of the electrons in the noble gas neon (atomic number 10). electronic configuration is defined as the distribution of electrons into the orbitals of an atom. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. Electron. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Chapter 5 Electrons In Atoms PowerPoint Presentation, free Rules Distribution Of Electrons fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. Although the distributions of electrons. Electron configurations of atoms follow a standard notation. the most common way to describe electron configurations is to write distributions in the spdf notation.. Rules Distribution Of Electrons.
From www.chem.fsu.edu
Electron Configurations Rules Distribution Of Electrons electronic configuration is defined as the distribution of electrons into the orbitals of an atom. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. the distribution of electrons in shells of the atomic orbitals of an. Rules Distribution Of Electrons.
From chem.libretexts.org
6.9 Electron Configurations and the Periodic Table Chemistry LibreTexts Rules Distribution Of Electrons fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Although the distributions of electrons. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. the most common way to describe electron configurations is to write distributions in the spdf notation. general rules for assigning electrons to. Rules Distribution Of Electrons.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration Rules Distribution Of Electrons general rules for assigning electrons to atomic orbitals. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. Electron configurations of atoms follow a standard notation. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. fluorine (atomic number 9) has only. Rules Distribution Of Electrons.
From www.scienceabc.com
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable? Rules Distribution Of Electrons An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. the electron. Rules Distribution Of Electrons.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Rules Distribution Of Electrons the most common way to describe electron configurations is to write distributions in the spdf notation. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. An. Rules Distribution Of Electrons.
From www.doubtnut.com
Summarise the rules for writing of distribution of electrons in variou Rules Distribution Of Electrons electronic configuration is defined as the distribution of electrons into the orbitals of an atom. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. the most common way to describe electron configurations is to write distributions in the spdf notation.. Rules Distribution Of Electrons.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Rules Distribution Of Electrons the most common way to describe electron configurations is to write distributions in the spdf notation. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. An. Rules Distribution Of Electrons.
From eduinput.com
RULES FOR THE DISTRIBUTION OF ELECTRONS Rules Distribution Of Electrons Although the distributions of electrons. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. the electron configuration of an element describes how electrons are distributed. Rules Distribution Of Electrons.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Rules Distribution Of Electrons the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons. Rules Distribution Of Electrons.
From www.vedantu.com
Distribution of Electrons in Different Orbits/Shells Learn Important Rules Distribution Of Electrons Electron configurations of atoms follow a standard notation. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. all we need to know. Rules Distribution Of Electrons.
From opentextbc.ca
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry Rules Distribution Of Electrons electronic configuration is defined as the distribution of electrons into the orbitals of an atom. Electron configurations of atoms follow a standard notation. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. the electron configuration of an element describes how. Rules Distribution Of Electrons.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write Rules Distribution Of Electrons the most common way to describe electron configurations is to write distributions in the spdf notation. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. Electron configurations of atoms follow a standard notation. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron.. Rules Distribution Of Electrons.
From www.studypool.com
SOLUTION Electronic configuration and rules for distribution of Rules Distribution Of Electrons All of the electrons in the noble gas neon (atomic number 10). An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. the most common way to describe electron configurations is to write distributions in the spdf notation. electronic configuration is defined as the distribution of electrons into the orbitals of an atom.. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Electron Configuration Notation with Atomic Structure Review Rules Distribution Of Electrons An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. general rules for assigning electrons to atomic orbitals. An atom consists of subatomic. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation. electronic configuration is defined as the. Rules Distribution Of Electrons.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry Rules Distribution Of Electrons the most common way to describe electron configurations is to write distributions in the spdf notation. An atom consists of subatomic. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. all we need to know is how many protons it has (and how many electrons, which is the same as the. Rules Distribution Of Electrons.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Rules Distribution Of Electrons All of the electrons in the noble gas neon (atomic number 10). Although the distributions of electrons. An atom consists of subatomic. general rules for assigning electrons to atomic orbitals. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. Electron configurations. Rules Distribution Of Electrons.
From www.thoughtco.com
Electron Configuration Chart Rules Distribution Of Electrons fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. All of. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Distribution of electrons in various shells. PowerPoint Rules Distribution Of Electrons general rules for assigning electrons to atomic orbitals. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. An atom consists of subatomic. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for. Rules Distribution Of Electrons.
From sciencenotes.org
List of Electron Configurations of Elements Rules Distribution Of Electrons fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Although the distributions of electrons. Electron configurations of atoms follow a standard notation. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. . Rules Distribution Of Electrons.
From www.studypool.com
SOLUTION Electronic configuration and rules for distribution of Rules Distribution Of Electrons electronic configuration is defined as the distribution of electrons into the orbitals of an atom. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. An atom consists of subatomic. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. the most common way to describe electron. Rules Distribution Of Electrons.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica Rules Distribution Of Electrons Although the distributions of electrons. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. general rules. Rules Distribution Of Electrons.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes Rules Distribution Of Electrons Although the distributions of electrons. the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. general rules for assigning electrons to atomic orbitals. An atom consists of subatomic. electronic configuration is defined as. Rules Distribution Of Electrons.
From chem.libretexts.org
3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts Rules Distribution Of Electrons the distribution of electrons in shells of the atomic orbitals of an atom is known as its electronic configuration. An atom consists of subatomic. the electron configuration of an element describes how electrons are distributed in its atomic orbitals. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. the most. Rules Distribution Of Electrons.
From www.slideserve.com
PPT Distribution of electrons in various shells. PowerPoint Rules Distribution Of Electrons All of the electrons in the noble gas neon (atomic number 10). Although the distributions of electrons. electronic configuration is defined as the distribution of electrons into the orbitals of an atom. general rules for assigning electrons to atomic orbitals. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. An atom consists. Rules Distribution Of Electrons.
From www.studypool.com
SOLUTION Electronic configuration and rules for distribution of Rules Distribution Of Electrons the most common way to describe electron configurations is to write distributions in the spdf notation. all we need to know is how many protons it has (and how many electrons, which is the same as the number of protons for a. fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. . Rules Distribution Of Electrons.
From gzscienceclassonline.weebly.com
1. Electron Configuration Rules Distribution Of Electrons fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. An atom consists of subatomic. the most common way to describe electron configurations is to write distributions in the spdf notation. All of the electrons in the noble gas neon (atomic number 10). An atom’s electrons exist in discrete atomic orbitals, and the atom’s. Rules Distribution Of Electrons.
From www.youtube.com
Summarise the rules for writing of distribution of electrons in various Rules Distribution Of Electrons general rules for assigning electrons to atomic orbitals. the most common way to describe electron configurations is to write distributions in the spdf notation. An atom’s electrons exist in discrete atomic orbitals, and the atom’s electron configuration can be. Although the distributions of electrons. All of the electrons in the noble gas neon (atomic number 10). Electron configurations. Rules Distribution Of Electrons.