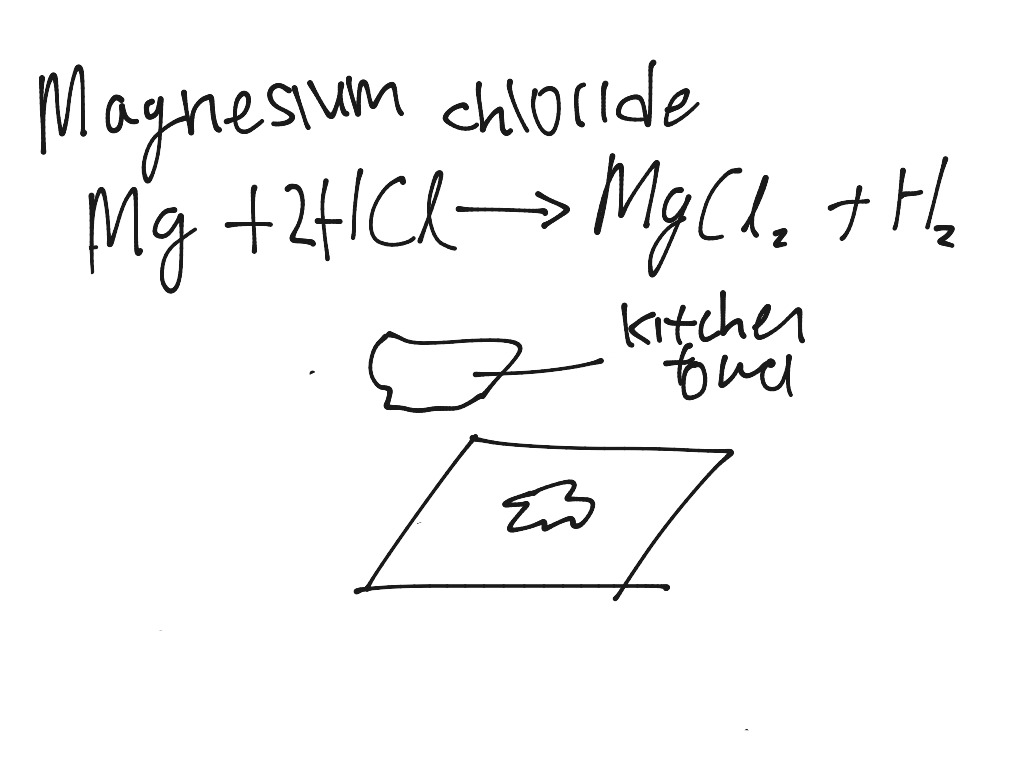
Molten Magnesium Chloride Formula . Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). (a) sketch the cell, label the anode. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Electrolysis separates the molten ionic compound into its. Anhydrous magnesium chloride is readily soluble in water. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. History.involved the reduction of molten magnesium chloride by metallic potassium. Magnesium chloride must be heated until it is molten before it will conduct electricity.
from www.showme.com
Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Anhydrous magnesium chloride is readily soluble in water. Magnesium chloride must be heated until it is molten before it will conduct electricity. Electrolysis separates the molten ionic compound into its. (a) sketch the cell, label the anode. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k History.involved the reduction of molten magnesium chloride by metallic potassium. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which.
ShowMe magnesium chloride
Molten Magnesium Chloride Formula Magnesium chloride must be heated until it is molten before it will conduct electricity. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. (a) sketch the cell, label the anode. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Magnesium chloride must be heated until it is molten before it will conduct electricity. Anhydrous magnesium chloride is readily soluble in water. Electrolysis separates the molten ionic compound into its. History.involved the reduction of molten magnesium chloride by metallic potassium. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k
From www.magnesiumchloridechina.com
Magnesium Chloride Powder Molten Magnesium Chloride Formula Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Anhydrous magnesium chloride is readily soluble in water. (a) sketch the cell, label the anode. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml. Molten Magnesium Chloride Formula.
From www.chegg.com
Solved Question 2 10 pts At 900°C titanium tetrachloride Molten Magnesium Chloride Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium chloride must be heated until it is molten before it will conduct electricity. Its solubility ranges. Molten Magnesium Chloride Formula.
From www.melford.co.uk
M240001000.0 Magnesium Chloride Hexahydrate, 1 Kilogram Molten Magnesium Chloride Formula History.involved the reduction of molten magnesium chloride by metallic potassium. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium chloride must be heated until it is molten before it will conduct electricity. (a) sketch the cell, label the anode. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per. Molten Magnesium Chloride Formula.
From www.youtube.com
Empirical Formula of Magnesium Chloride LAB YouTube Molten Magnesium Chloride Formula Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Magnesium chloride must be heated until it is molten before it will conduct electricity.. Molten Magnesium Chloride Formula.
From www.cbsetuts.com
What is the chemical formula of magnesium chloride? CBSE Tuts Molten Magnesium Chloride Formula Electrolysis separates the molten ionic compound into its. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. History.involved the reduction of molten magnesium chloride by metallic potassium. (a) sketch the cell, label the anode. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Its solubility ranges from 52.9 g per. Molten Magnesium Chloride Formula.
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra Molten Magnesium Chloride Formula Electrolysis separates the molten ionic compound into its. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). (a) sketch the cell, label the anode. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. History.involved the reduction of molten magnesium. Molten Magnesium Chloride Formula.
From ar.inspiredpencil.com
Mass To Mole Formula Molten Magnesium Chloride Formula Magnesium chloride must be heated until it is molten before it will conduct electricity. Electrolysis separates the molten ionic compound into its. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. History.involved the reduction of molten magnesium chloride by metallic. Molten Magnesium Chloride Formula.
From www.youtube.com
Write the chemical formula of the following compounds (a)Magnesium Molten Magnesium Chloride Formula (a) sketch the cell, label the anode. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Magnesium chloride must be heated until it is molten before it will conduct electricity. Electrolysis separates the molten ionic compound into its. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml. Molten Magnesium Chloride Formula.
From chemistry291.blogspot.com
Chemical Formula for Sodium ChlorideSodium Chloride Formula Molten Magnesium Chloride Formula Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Anhydrous magnesium chloride is readily soluble in water. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k (a) sketch the cell, label the anode. History.involved the reduction of molten magnesium chloride by metallic potassium. In. Molten Magnesium Chloride Formula.
From jbdizkkrki.blogspot.com
Magnesium Chloride Formula Unit The Structure Of Matter Section 1 Molten Magnesium Chloride Formula Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Electrolysis separates the molten ionic compound into its. Anhydrous magnesium chloride is readily soluble in water. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). (a) sketch the cell, label the anode. History.involved the reduction of molten magnesium. Molten Magnesium Chloride Formula.
From www.numerade.com
SOLVED Magnesium and chlorine can be made by the electrolysis of Molten Magnesium Chloride Formula In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Electrolysis separates the molten ionic compound into its. Anhydrous magnesium chloride is readily soluble in water. Its solubility ranges from 52.9 g per 100 ml at 273 k,. Molten Magnesium Chloride Formula.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Molten Magnesium Chloride Formula (a) sketch the cell, label the anode. Electrolysis separates the molten ionic compound into its. History.involved the reduction of molten magnesium chloride by metallic potassium. Anhydrous magnesium chloride is readily soluble in water. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Molten magnesium chloride boils at a temperature. Molten Magnesium Chloride Formula.
From www.numerade.com
SOLVEDMagnesium metal is produced by electrolysis of molten magnesium Molten Magnesium Chloride Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Anhydrous magnesium chloride is readily soluble in water. History.involved the reduction of molten magnesium chloride by metallic potassium. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. (a) sketch the. Molten Magnesium Chloride Formula.
From www.glentham.com
Magnesium chloride, anhydrous (CAS 7786303) Glentham Life Sciences Molten Magnesium Chloride Formula Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Electrolysis separates the molten ionic compound into its. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k History.involved the reduction of molten magnesium chloride. Molten Magnesium Chloride Formula.
From www.youtube.com
Write the chemical formula of Magnesium chloride YouTube Molten Magnesium Chloride Formula Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Magnesium chloride must be heated until it is molten before it will conduct electricity. History.involved the reduction of molten magnesium chloride by metallic potassium. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. (a) sketch. Molten Magnesium Chloride Formula.
From brainly.in
10 (a) A student investigates the electrolysis of molten magnesium Molten Magnesium Chloride Formula (a) sketch the cell, label the anode. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Magnesium chloride must be heated until it is molten before it will conduct electricity. Anhydrous magnesium chloride is readily soluble in. Molten Magnesium Chloride Formula.
From webapi.bu.edu
😝 Write the formula for the compound magnesium oxide. What is the Molten Magnesium Chloride Formula Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Electrolysis separates the molten ionic compound into its. (a) sketch the cell, label the anode. Magnesium chloride must be heated until it is molten before it will conduct. Molten Magnesium Chloride Formula.
From signalticket9.pythonanywhere.com
Unbelievable Magnesium Chloride Balanced Equation Maths Formulas For Molten Magnesium Chloride Formula Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Electrolysis separates the molten ionic compound into its. History.involved the reduction. Molten Magnesium Chloride Formula.
From brainly.in
Using criss cross method write the formula for Magnesium Chloride Molten Magnesium Chloride Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Anhydrous magnesium chloride is readily soluble in water. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium chloride must be heated until it is molten. Molten Magnesium Chloride Formula.
From www.researchgate.net
study of electrochemical deoxidation of commercially pure Molten Magnesium Chloride Formula Magnesium chloride must be heated until it is molten before it will conduct electricity. Electrolysis separates the molten ionic compound into its. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k (a) sketch the cell, label the anode. Anhydrous magnesium chloride is readily soluble in water. In the electrolysis. Molten Magnesium Chloride Formula.
From brainly.in
what is the chemical formula of carbon dioxide for examples Molten Magnesium Chloride Formula In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Electrolysis separates the molten ionic compound into its.. Molten Magnesium Chloride Formula.
From www.chegg.com
Solved (a) Molten magnesium chloride is electrolysed in a Molten Magnesium Chloride Formula Anhydrous magnesium chloride is readily soluble in water. Electrolysis separates the molten ionic compound into its. History.involved the reduction of molten magnesium chloride by metallic potassium. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. (a) sketch the cell, label the anode. Magnesium chloride must be heated until it is molten. Molten Magnesium Chloride Formula.
From dongachem.vn
Magie clorua là gì? Tính chất, điều chế và ứng dụng của MgCl2 Molten Magnesium Chloride Formula Anhydrous magnesium chloride is readily soluble in water. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Electrolysis separates the molten ionic compound into its. Magnesium chloride must be heated until it is molten before. Molten Magnesium Chloride Formula.
From www.numerade.com
SOLVED Magnesium metal reacts with chlorine gas, Cl2, to produce Molten Magnesium Chloride Formula In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Electrolysis separates the molten ionic compound into its. Molten magnesium chloride boils at a temperature of 1685. Molten Magnesium Chloride Formula.
From www.numerade.com
SOLVED How many formula units make up 20.0 gg of magnesium chloride Molten Magnesium Chloride Formula Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Anhydrous magnesium chloride is readily soluble in water. Magnesium chloride must be heated until it is molten before it will conduct electricity. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Its solubility ranges from 52.9 g. Molten Magnesium Chloride Formula.
From studdy.in
4. Write the chemical formulae of the following Studdy Molten Magnesium Chloride Formula Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Anhydrous magnesium chloride is readily soluble in water. Magnesium chloride consists of. Molten Magnesium Chloride Formula.
From www.showme.com
ShowMe magnesium chloride Molten Magnesium Chloride Formula Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. (a) sketch the cell, label the anode. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride must be heated until it is molten before it will conduct electricity. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g. Molten Magnesium Chloride Formula.
From molekula.com
Purchase Magnesium chloride hexahydrate [7791186] online • Catalog Molten Magnesium Chloride Formula Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Anhydrous magnesium chloride is readily soluble in water. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium chloride must be heated until it. Molten Magnesium Chloride Formula.
From jkenterprises.com.pk
Magnesium chloride, pure J K Enterprises Chemical J K Enterprises Molten Magnesium Chloride Formula (a) sketch the cell, label the anode. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Electrolysis separates the molten ionic. Molten Magnesium Chloride Formula.
From sujatanutripharma.com
Magnesium Chloride Hexahydrate Sujata Nutri Pharma Molten Magnesium Chloride Formula Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). History.involved the reduction of molten magnesium chloride by metallic potassium. (a) sketch the cell, label the anode. Magnesium metal is produced by the electrolysis of molten magnesium chloride using inert electrodes. Electrolysis separates the molten ionic compound into its. In the electrolysis of molten mgcl2 using graphite electrodes, the. Molten Magnesium Chloride Formula.
From www.youtube.com
3.26 electrolysis of magnesium chloride YouTube Molten Magnesium Chloride Formula (a) sketch the cell, label the anode. Anhydrous magnesium chloride is readily soluble in water. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. History.involved the reduction of molten magnesium chloride by metallic potassium. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Magnesium metal is produced by the. Molten Magnesium Chloride Formula.
From inci.guide
Magnesium Chloride Ingredient INCIGuide Molten Magnesium Chloride Formula Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k (a) sketch the cell, label the anode. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. History.involved. Molten Magnesium Chloride Formula.
From kunduz.com
[ANSWERED] An electrolytic cell is prepared for the purpose of Molten Magnesium Chloride Formula In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3 g per 100 ml at 293 k Electrolysis separates the molten ionic compound into its.. Molten Magnesium Chloride Formula.
From www.thomassci.com
Magnesium Chloride, hexahydrate Molten Magnesium Chloride Formula History.involved the reduction of molten magnesium chloride by metallic potassium. Magnesium chloride consists of magnesium cations (mg²⁺) and chloride anions (cl⁻). Electrolysis separates the molten ionic compound into its. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards. Molten Magnesium Chloride Formula.
From www.youtube.com
Electrolysis of molten magnesium chloride results in the formation of 0 Molten Magnesium Chloride Formula In the electrolysis of molten mgcl2 using graphite electrodes, the electrode which is positively charged and towards which. (a) sketch the cell, label the anode. Anhydrous magnesium chloride is readily soluble in water. Molten magnesium chloride boils at a temperature of 1685 k, converting into vapor form. Its solubility ranges from 52.9 g per 100 ml at 273 k, 54.3. Molten Magnesium Chloride Formula.