Hydrogen Electrode For Class 12Th . For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. How would you determine the standard electrode potential of the system mg2+1 mg?ans: The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and
from www.vecteezy.com
This is a reference electrode to which all electrodes are calculated in terms of electrode potential. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). How would you determine the standard electrode potential of the system mg2+1 mg?ans: Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and
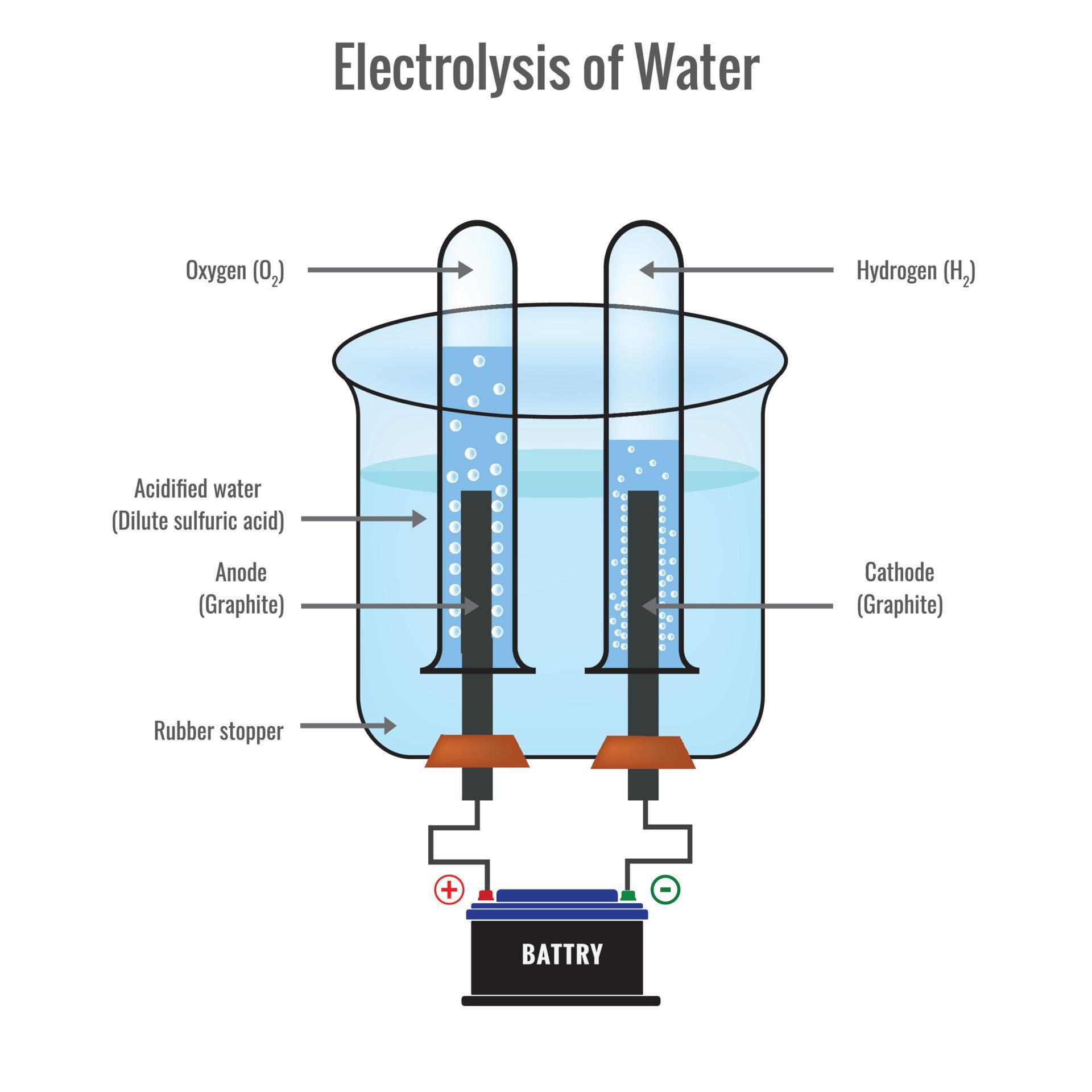
Electrolysis of water forming Hydrogen and Oxygen vector illustration
Hydrogen Electrode For Class 12Th A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). How would you determine the standard electrode potential of the system mg2+1 mg?ans: A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image Hydrogen Electrode For Class 12Th The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. This is a reference electrode to which all electrodes are calculated in terms of. Hydrogen Electrode For Class 12Th.
From www.vecteezy.com
Electrolysis of water forming Hydrogen and Oxygen vector illustration Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). A cell, consisting of mg | mgso 4 (aq 1 m). Hydrogen Electrode For Class 12Th.
From www.youtube.com
03 Electrochemistry Electrode Potaintial Standard Hydrogen Electrode Hydrogen Electrode For Class 12Th The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode. Hydrogen Electrode For Class 12Th.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry Hydrogen Electrode For Class 12Th How would you determine the standard electrode potential of the system mg2+1 mg?ans: Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. The standard electrode potential of mg 2+ | mg can be measured with respect. Hydrogen Electrode For Class 12Th.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Hydrogen Electrode For Class 12Th Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and How would you determine the standard electrode potential of the system mg2+1 mg?ans:. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Standard Hydrogen Electrode Electrochemistry Chemistry Class 12 Hydrogen Electrode For Class 12Th How would you determine the standard electrode potential of the system mg2+1 mg?ans: A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode. Hydrogen Electrode For Class 12Th.
From www.youtube.com
manak hydrogen electrode//मानक हाइड्रोजन इलेक्ट्रोड //unit3//class Hydrogen Electrode For Class 12Th For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be measured with respect to. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Electrochemistry (L03) Standard Hydrogen Electrode (SHE) Theory Hydrogen Electrode For Class 12Th How would you determine the standard electrode potential of the system mg2+1 mg?ans: This is a reference electrode to which all electrodes are calculated in terms of electrode potential. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and The standard electrode. Hydrogen Electrode For Class 12Th.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode. Hydrogen Electrode For Class 12Th.
From scienceinfo.com
What is Standard Hydrogen Electrode? Hydrogen Electrode For Class 12Th A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) |. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Electrochemistry 12th Class Standard Hydrogen Electrode Electrode Hydrogen Electrode For Class 12Th How would you determine the standard electrode potential of the system mg2+1 mg?ans: The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A. Hydrogen Electrode For Class 12Th.
From www.youtube.com
STANDARD HYDROGEN ELECTRODE (SHE)मानक इलेक्ट्रोड विभव Class 12th Hydrogen Electrode For Class 12Th Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). For more such engaging content, download iprep and learn unlimitedplay store. Hydrogen Electrode For Class 12Th.
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Hydrogen Electrode For Class 12Th A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm). Hydrogen Electrode For Class 12Th.
From saylordotorg.github.io
Electrochemistry Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Decoding of Standard Hydrogen electrode. SHE for class 12, JEE, NEET Hydrogen Electrode For Class 12Th The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). How would you determine the standard electrode potential of the system mg2+1 mg?ans: For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. This. Hydrogen Electrode For Class 12Th.
From www.youtube.com
ELECTROCHEMISTRY PART 2 // STANDARD HYDROGEN ELECTRODE //CLASS 12TH Hydrogen Electrode For Class 12Th A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Standard Hydrogen Electrode 35 Electrochemistry Class 12, NEET Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. How would you determine the standard electrode potential of the system mg2+1 mg?ans: Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell. Hydrogen Electrode For Class 12Th.
From www.youtube.com
मानक हाइड्रोजन इलेक्ट्रोड SHE standard hydrogen electrode ।12th Hydrogen Electrode For Class 12Th For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Electrochemistry 5 Standard hydrogen electrode YouTube Hydrogen Electrode For Class 12Th Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. The standard electrode potential of mg 2+ | mg can be measured with respect to. Hydrogen Electrode For Class 12Th.
From www.youtube.com
CLASS 12TH ELECTROCHEMISTRY Standard Hydrogen Electrode YouTube Hydrogen Electrode For Class 12Th For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. The standard electrode potential of mg 2+ | mg can be measured with respect. Hydrogen Electrode For Class 12Th.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Hydrogen Electrode For Class 12Th For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. How would you determine the standard electrode potential of the system mg2+1 mg?ans: Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Hydrogen Electrode For Class 12Th Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be. Hydrogen Electrode For Class 12Th.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and How would you determine the standard electrode potential of the system mg2+1 mg?ans: Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black.. Hydrogen Electrode For Class 12Th.
From www.youtube.com
standard hydrogen electrode YouTube Hydrogen Electrode For Class 12Th A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and How would you determine the standard electrode potential of the system mg2+1 mg?ans: For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. Hydrogen electrode. Hydrogen Electrode For Class 12Th.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Hydrogen Electrode For Class 12Th How would you determine the standard electrode potential of the system mg2+1 mg?ans: A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode,. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Standard Hydrogen Electrode Electrochemistry class 12th YouTube Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated. Hydrogen Electrode For Class 12Th.
From www.doubtnut.com
Describe the construction and working of the standard hydrogen electr Hydrogen Electrode For Class 12Th Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). For more such engaging content, download iprep and learn unlimitedplay store. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Class 12th standard hydrogen electrode (electrochemistry) YouTube Hydrogen Electrode For Class 12Th For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode. Hydrogen Electrode For Class 12Th.
From askfilo.com
Standard hydrogen electrode (SHE) It is used as reference electrode who.. Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode. Hydrogen Electrode For Class 12Th.
From mavink.com
Hydrogen Electrode Diagram Hydrogen Electrode For Class 12Th A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. For more such engaging content, download iprep and learn unlimitedplay store. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Standard Hydrogen Electrode(SHE)\\ class 12th unit III electrochemistry Hydrogen Electrode For Class 12Th How would you determine the standard electrode potential of the system mg2+1 mg?ans: A cell, consisting of mg | mgso 4 (aq 1 m) as the anode and Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. This is a reference electrode to which all electrodes are calculated in terms of electrode potential.. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Standard hydrogen electrode Diagram of S.H.E Electrochemistry Hydrogen Electrode For Class 12Th Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. How would you determine the standard electrode potential of the system mg2+1 mg?ans: A cell. Hydrogen Electrode For Class 12Th.
From www.youtube.com
Standard Hydrogen Electrode (SHE) Normal Hydrogen Electrode (NHE Hydrogen Electrode For Class 12Th This is a reference electrode to which all electrodes are calculated in terms of electrode potential. The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). A cell, consisting of mg | mgso 4 (aq 1 m). Hydrogen Electrode For Class 12Th.
From monomole.com
Standard hydrogen electrode Mono Mole Hydrogen Electrode For Class 12Th For more such engaging content, download iprep and learn unlimitedplay store 𑗅 app. How would you determine the standard electrode potential of the system mg2+1 mg?ans: The standard electrode potential of mg 2+ | mg can be measured with respect to the standard hydrogen electrode, represented by pt (s), h 2(g) (1 atm) | h + (aq) (1 m). Hydrogen. Hydrogen Electrode For Class 12Th.
From byjus.com
What is standard hydrogen electrode? Hydrogen Electrode For Class 12Th A cell will be set up consisting of mg/mgso 4 (1 m) as one electrode and standard. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. Hydrogen electrode (nhe), consists of platinum wire, carrying platinum foil coated with finely divided platinum black. A cell, consisting of mg | mgso 4 (aq 1 m). Hydrogen Electrode For Class 12Th.