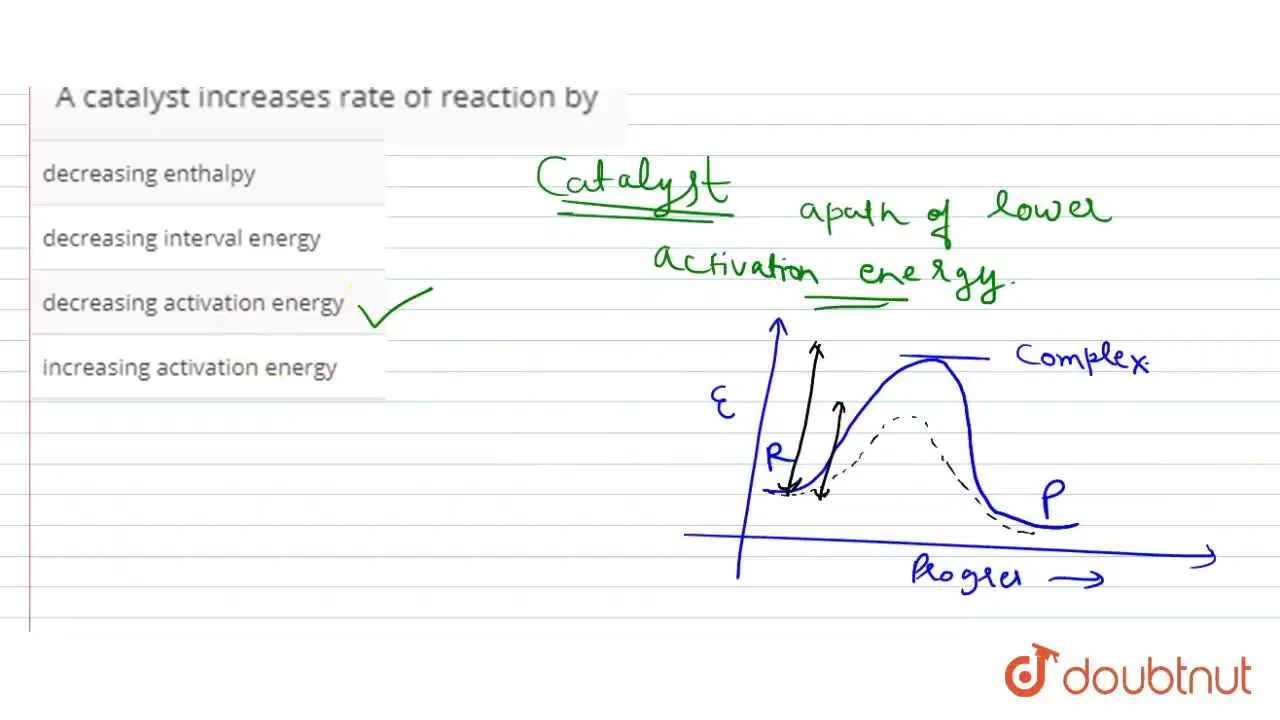
A Catalyst Is A Substance That Increases The Rate Of A Reaction By . catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. product a substance formed in a chemical reaction. this page explains how adding a catalyst affects the rate of a reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. A very small amount of catalyst is required to alter the reaction rate. It assumes familiarity with basic concepts in the collision theory of.
from www.doubtnut.com
A very small amount of catalyst is required to alter the reaction rate. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. this page explains how adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. product a substance formed in a chemical reaction. It assumes familiarity with basic concepts in the collision theory of. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate.
A catalyst increases rate of reaction by
A Catalyst Is A Substance That Increases The Rate Of A Reaction By catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. product a substance formed in a chemical reaction. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. this page explains how adding a catalyst affects the rate of a reaction. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. A very small amount of catalyst is required to alter the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. It assumes familiarity with basic concepts in the collision theory of.
From slideplayer.com
Equilibrium Chapter ppt download A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. A very small amount of catalyst is required to alter the reaction rate. catalysis is a process of increasing the rate of a chemical reaction. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 A Catalyst Is A Substance That Increases The Rate Of A Reaction By It assumes familiarity with basic concepts in the collision theory of. this page explains how adding a catalyst affects the rate of a reaction. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate.. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From slideplayer.com
REACTION RATES. ppt download A Catalyst Is A Substance That Increases The Rate Of A Reaction By It assumes familiarity with basic concepts in the collision theory of. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Only a very small mass of catalyst is needed to increase the rate. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From gioaralqv.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction In Such A Manner A Catalyst Is A Substance That Increases The Rate Of A Reaction By A very small amount of catalyst is required to alter the reaction rate. this page explains how adding a catalyst affects the rate of a reaction. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.dreamstime.com
Catalyst Substance that Increases the Rate of a Chemical Reaction A Catalyst Is A Substance That Increases The Rate Of A Reaction By catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. product a substance formed in a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. this page explains how adding a catalyst affects the. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.chegg.com
Solved A catalyst is a substance which increases the rate of A Catalyst Is A Substance That Increases The Rate Of A Reaction By A very small amount of catalyst is required to alter the reaction rate. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. catalysis is a process of increasing the rate of a chemical. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From byjus.com
How does a catalyst increase the rate of a reaction? A Catalyst Is A Substance That Increases The Rate Of A Reaction By A very small amount of catalyst is required to alter the reaction rate. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. product a substance formed in a. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From slideplayer.com
Shock Tube Catalytic Activity Experiments ppt download A Catalyst Is A Substance That Increases The Rate Of A Reaction By Only a very small mass of catalyst is needed to increase the rate of a. this page explains how adding a catalyst affects the rate of a reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the greater the frequency of successful collisions between reactant particles, the greater the. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.slideshare.net
Biology 2.4 A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. product a substance formed in a chemical reaction. Only a very small mass of catalyst is needed to increase the rate of a. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By Only a very small mass of catalyst is needed to increase the rate of a. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A very small amount. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From fyorswrmb.blob.core.windows.net
How Does Catalyst Affect The Rate Of Chemical Reaction at Jermaine A Catalyst Is A Substance That Increases The Rate Of A Reaction By product a substance formed in a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A very small amount of catalyst is required to alter the reaction rate. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. Only a. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From fyoppxate.blob.core.windows.net
Catalytic Use Meaning at Norman Adair blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. product a substance formed in a chemical reaction. Only a very small mass of catalyst is needed. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.shutterstock.com
Catalyst Substance That Increases Rate Chemical Stock Vector (Royalty A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. this. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Factors that Affect Rate of Reaction PowerPoint Presentation A Catalyst Is A Substance That Increases The Rate Of A Reaction By product a substance formed in a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. temperature, reactant concentration, size of solid reactant particles (surface. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From answerhappy.com
A catalyst is a substance that increases the rate of reaction but does A Catalyst Is A Substance That Increases The Rate Of A Reaction By A very small amount of catalyst is required to alter the reaction rate. product a substance formed in a chemical reaction. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. this page explains how. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By It assumes familiarity with basic concepts in the collision theory of. A very small amount of catalyst is required to alter the reaction rate. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. this page explains how adding a catalyst affects the rate of a reaction. catalyst, in chemistry, any substance. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From exohgcpop.blob.core.windows.net
A Catalyst Increases The Rate Of Reaction As It at Gladys McCoy blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By product a substance formed in a chemical reaction. It assumes familiarity with basic concepts in the collision theory of. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. A very small amount of catalyst is required to alter the reaction rate. this page explains how adding a catalyst. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From exoeaikmf.blob.core.windows.net
Catalyst Increases The Rate Of The Reaction By at Lisa Hardaway blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A very small amount of catalyst is required to alter the reaction rate. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From guidepartrefractor.z21.web.core.windows.net
Energy Diagram Catalyzed Reaction A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. product a substance formed in a chemical reaction. It. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.dreamstime.com
Catalyst Substance that Increases the Rate of a Chemical Reaction A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. It assumes familiarity with basic concepts in the collision theory of. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Only a very small mass of catalyst is needed to increase the rate. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.doubtnut.com
A catalyst increases rate of reaction by A Catalyst Is A Substance That Increases The Rate Of A Reaction By product a substance formed in a chemical reaction. this page explains how adding a catalyst affects the rate of a reaction. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. catalysis is. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From exyxozbyo.blob.core.windows.net
How Are Enzymes And Chemical Catalysts Different at Randy Johnson blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By It assumes familiarity with basic concepts in the collision theory of. product a substance formed in a chemical reaction. Only a very small mass of catalyst is needed to increase the rate of a. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. this page explains how adding a catalyst affects. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. It assumes familiarity with basic concepts in the collision theory of. product a substance formed in a chemical reaction. A very small amount of catalyst is required to alter the reaction rate. Only a very small mass of catalyst is. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint A Catalyst Is A Substance That Increases The Rate Of A Reaction By Only a very small mass of catalyst is needed to increase the rate of a. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A very small amount of catalyst is required to alter the reaction rate. product a substance formed in a chemical. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii A Catalyst Is A Substance That Increases The Rate Of A Reaction By catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. product a substance formed in a chemical reaction. this page explains how adding a catalyst affects the. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From answerhappy.com
A catalyst is a substance that increases the rate of reaction but does A Catalyst Is A Substance That Increases The Rate Of A Reaction By catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A very small amount of catalyst is required to alter the reaction rate. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. It assumes familiarity with basic concepts in the collision theory of.. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From loevndwvy.blob.core.windows.net
A Catalyst Increase The Rate Of A Reaction By at Carey Rains blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of. product a substance formed in a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. the greater the frequency of successful collisions between. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 A Catalyst Is A Substance That Increases The Rate Of A Reaction By the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A very small amount of catalyst is required to alter the reaction rate. It assumes familiarity with basic concepts in the collision theory of. product a substance formed in a chemical reaction. catalyst, in chemistry, any substance that increases the rate of. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.pinterest.com
Catalyst definition A substance that speeds up a chemical reaction by A Catalyst Is A Substance That Increases The Rate Of A Reaction By catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. It assumes familiarity with basic concepts in the collision theory of. A very small amount of catalyst is required to alter the reaction rate. product a substance formed in a chemical reaction. the greater. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.numerade.com
SOLVED 1 Which best defines 'reaction rate'? a) Concentration change A Catalyst Is A Substance That Increases The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. product a substance formed in a chemical reaction. A very small amount of catalyst is required to alter the reaction rate. the greater the frequency. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From ppt-online.org
Rates of reaction презентация онлайн A Catalyst Is A Substance That Increases The Rate Of A Reaction By this page explains how adding a catalyst affects the rate of a reaction. It assumes familiarity with basic concepts in the collision theory of. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From www.slideserve.com
PPT Factors Affecting the Rate of a Chemical Reaction PowerPoint A Catalyst Is A Substance That Increases The Rate Of A Reaction By It assumes familiarity with basic concepts in the collision theory of. A very small amount of catalyst is required to alter the reaction rate. product a substance formed in a chemical reaction. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. this page. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From loeljyzzv.blob.core.windows.net
Catalyst Chemical Reaction Rate at Leandro Jordan blog A Catalyst Is A Substance That Increases The Rate Of A Reaction By catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Only a very small mass of catalyst is needed to increase the rate of a. It assumes familiarity with basic concepts in the collision theory of. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From slideplayer.com
Empirical Effect of Catalysis ppt download A Catalyst Is A Substance That Increases The Rate Of A Reaction By Only a very small mass of catalyst is needed to increase the rate of a. this page explains how adding a catalyst affects the rate of a reaction. the greater the frequency of successful collisions between reactant particles, the greater the reaction rate. catalyst, in chemistry, any substance that increases the rate of a reaction without itself. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.
From slideplayer.com
Factors Affecting Rates of Chemical Reactions ppt download A Catalyst Is A Substance That Increases The Rate Of A Reaction By temperature, reactant concentration, size of solid reactant particles (surface area) and catalysts can all affect the reaction rate. Only a very small mass of catalyst is needed to increase the rate of a. catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. this. A Catalyst Is A Substance That Increases The Rate Of A Reaction By.