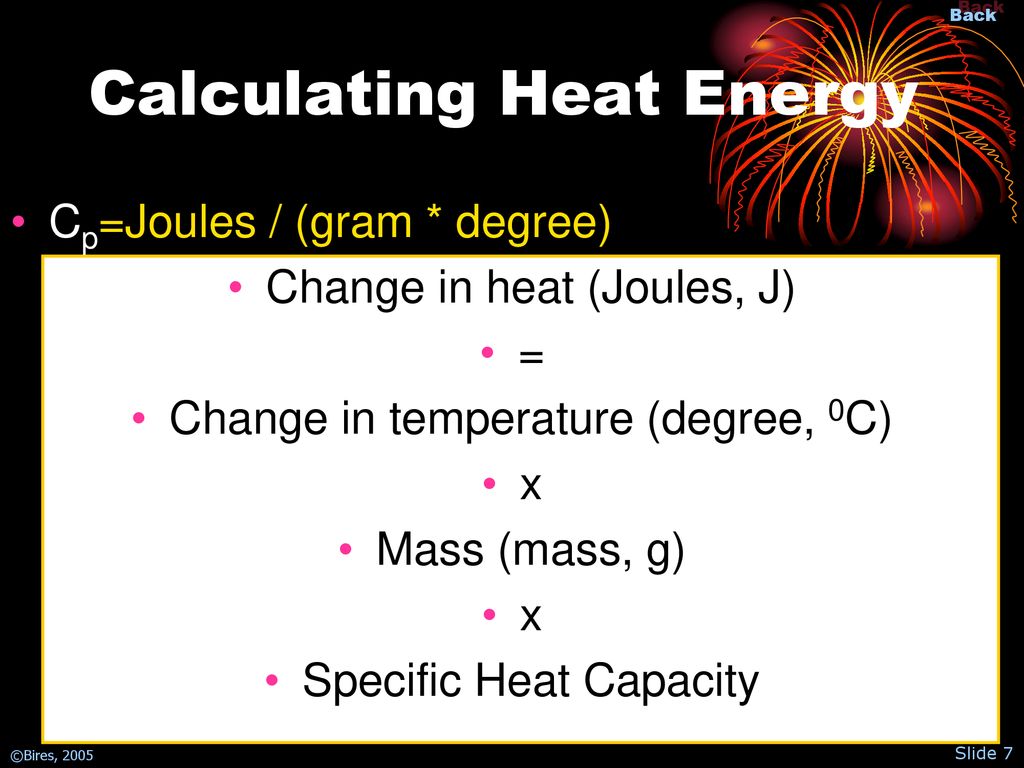
Heat Capacity Definition Joules . It may also be expressed as j/kg·k. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. This means that it takes 4,200 j to raise the. It is usually expressed as calories per degree in terms of the. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. In si units, specific heat capacity (symbol: The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. It is measured in joules per kelvin and given by. Heat capacity, ratio of heat absorbed by a material to the temperature change. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. A substance with a small heat. The temperature change (∆t) in the.
from slideplayer.com
It may also be expressed as j/kg·k. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. In si units, specific heat capacity (symbol: This means that it takes 4,200 j to raise the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It is usually expressed as calories per degree in terms of the. It is measured in joules per kelvin and given by.
Chapter 16 Reaction Energy ppt download
Heat Capacity Definition Joules Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The temperature change (∆t) in the. This means that it takes 4,200 j to raise the. In si units, specific heat capacity (symbol: In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. It is usually expressed as calories per degree in terms of the. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). It is measured in joules per kelvin and given by. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Heat capacity, ratio of heat absorbed by a material to the temperature change. It may also be expressed as j/kg·k. A substance with a small heat. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt.
From www.slideserve.com
PPT SPECIFIC HEAT CAPACITY PowerPoint Presentation, free download Heat Capacity Definition Joules C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. It is measured in joules per kelvin and given by. The temperature change (∆t) in the. Another unit of specific heat is calories per gram per. Heat Capacity Definition Joules.
From www.youtube.com
What is Joules law Definition and Formula of Joules law Joules Law Heat Capacity Definition Joules It is usually expressed as calories per degree in terms of the. This means that it takes 4,200 j to raise the. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt.. Heat Capacity Definition Joules.
From slideplayer.com
Specific Heat Part ppt download Heat Capacity Definition Joules The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. A substance with a small heat. Heat capacity, ratio of heat absorbed by a material to the temperature change.. Heat Capacity Definition Joules.
From www.geeksforgeeks.org
Heat Capacity Definition, Formula, Unit, Examples, FAQs Heat Capacity Definition Joules In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. Heat capacity, ratio of heat absorbed by a material to the temperature change. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The specific heat capacity of water is 4,200 joules per kilogram per. Heat Capacity Definition Joules.
From slideplayer.com
Key Plumbing Principles ppt download Heat Capacity Definition Joules Heat capacity, ratio of heat absorbed by a material to the temperature change. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. This means that it takes 4,200 j to raise the. A substance with. Heat Capacity Definition Joules.
From slideplayer.com
Specific Heat Capacity ppt download Heat Capacity Definition Joules In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). It is usually expressed as calories per degree in terms of the. A substance with a small heat. In si units, specific heat capacity (symbol: This. Heat Capacity Definition Joules.
From www.youtube.com
Joule's Law Example 1 (heating water) YouTube Heat Capacity Definition Joules A substance with a small heat. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It is measured in joules. Heat Capacity Definition Joules.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Definition Joules C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. A substance with a small heat. It may also be expressed as j/kg·k. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The temperature change (∆t) in the. Heat capacity is the measurable physical quantity. Heat Capacity Definition Joules.
From www.slideserve.com
PPT Properties of Materials PowerPoint Presentation, free download Heat Capacity Definition Joules In si units, specific heat capacity (symbol: Heat capacity, ratio of heat absorbed by a material to the temperature change. This means that it takes 4,200 j to raise the. A substance with a small heat. It is usually expressed as calories per degree in terms of the. Heat capacity is the measurable physical quantity that characterizes the amount of. Heat Capacity Definition Joules.
From www.slideserve.com
PPT THERMOCHEMISTRY Thermodynamics The study of Heat and Work and Heat Capacity Definition Joules It is measured in joules per kelvin and given by. This means that it takes 4,200 j to raise the. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. Heat capacity, ratio of heat absorbed by a material to the temperature change. The heat capacity (c) of a body of matter is the. Heat Capacity Definition Joules.
From www.youtube.com
Specific heat capacity YouTube Heat Capacity Definition Joules The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. It may also be expressed as j/kg·k. It is usually expressed as calories per degree in terms of the. Heat capacity, ratio. Heat Capacity Definition Joules.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Definition Joules In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. This means that. Heat Capacity Definition Joules.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow Heat Capacity Definition Joules A substance with a small heat. It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The temperature change (∆t) in the. In si units, specific heat capacity (symbol: Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change. Heat Capacity Definition Joules.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Heat Capacity Definition Joules This means that it takes 4,200 j to raise the. In si units, specific heat capacity (symbol: The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. It is usually expressed as calories per degree in terms of the. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to. Heat Capacity Definition Joules.
From www.youtube.com
Unit of heat energy SI unit of heat CGS unit of heat Joule Heat Capacity Definition Joules C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. In si units, specific heat capacity (symbol: The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature. Heat Capacity Definition Joules.
From slideplayer.com
Specific Heat Part ppt download Heat Capacity Definition Joules C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. The temperature change (∆t) in the. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. The heat capacity (c) of a body of matter is the quantity of. Heat Capacity Definition Joules.
From slideplayer.com
Heat Energy Heat (thermal) energy is simply a type of energy ppt Heat Capacity Definition Joules Heat capacity, ratio of heat absorbed by a material to the temperature change. In si units, specific heat capacity (symbol: It is usually expressed as calories per degree in terms of the. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The heat capacity (c) of a body of matter is the quantity. Heat Capacity Definition Joules.
From slideshare.net
Heat Capacity Heat Capacity Definition Joules It is usually expressed as calories per degree in terms of the. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. This means that it takes 4,200 j to raise the. It may also. Heat Capacity Definition Joules.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Definition Joules Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. It is usually expressed as calories per degree in terms of the. Heat capacity, ratio of heat absorbed by a material to the temperature change. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. The temperature change. Heat Capacity Definition Joules.
From jahamattbrown.blogspot.com
Specific Heat Capacity Definition Matt Brown Heat Capacity Definition Joules The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. C) is the amount of heat. Heat Capacity Definition Joules.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types Heat Capacity Definition Joules Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It is measured in joules per kelvin and given by. It is usually expressed as calories per degree in. Heat Capacity Definition Joules.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube Heat Capacity Definition Joules The temperature change (∆t) in the. In si units, specific heat capacity (symbol: In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It is usually expressed as calories per degree in terms. Heat Capacity Definition Joules.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video Heat Capacity Definition Joules Heat capacity, ratio of heat absorbed by a material to the temperature change. It may also be expressed as j/kg·k. In si units, specific heat capacity (symbol: In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. C) is the amount of heat in joules required to raise 1 gram of. Heat Capacity Definition Joules.
From slideplayer.com
Section Thermal Energy Transfer ppt download Heat Capacity Definition Joules Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. It is usually expressed as calories per degree in terms of the. It is measured in joules per kelvin and given by. Heat capacity, ratio of heat absorbed by a material to the temperature change. The temperature change (∆t) in the. Heat capacity is. Heat Capacity Definition Joules.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Heat Capacity Definition Joules This means that it takes 4,200 j to raise the. The temperature change (∆t) in the. It is measured in joules per kelvin and given by. It may also be expressed as j/kg·k. Heat capacity, ratio of heat absorbed by a material to the temperature change. C) is the amount of heat in joules required to raise 1 gram of. Heat Capacity Definition Joules.
From www.youtube.com
The heat capacity of a substance is the amount of energy, in joules (J Heat Capacity Definition Joules The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. A substance with a small heat. This means that it takes 4,200 j to raise the. It may also be expressed as. Heat Capacity Definition Joules.
From studymind.co.uk
Heat Capacity Study Mind Heat Capacity Definition Joules Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The temperature change (∆t) in the. Heat capacity, ratio of heat absorbed by a material to the temperature change. This means that. Heat Capacity Definition Joules.
From www.youtube.com
Specific Heat Capacity Solving for Joules YouTube Heat Capacity Definition Joules This means that it takes 4,200 j to raise the. A substance with a small heat. Heat capacity, ratio of heat absorbed by a material to the temperature change. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. C) is the amount of heat in joules required to raise 1. Heat Capacity Definition Joules.
From www.youtube.com
Joules, Food Calories, & Kilojoules Unit Conversion With Heat Energy Heat Capacity Definition Joules A substance with a small heat. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). This means that it takes 4,200 j to raise the. In si units, specific heat capacity (symbol: Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. It is measured in joules per. Heat Capacity Definition Joules.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4496534 Heat Capacity Definition Joules The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. This means that it takes 4,200 j to raise the. Heat capacity is the measurable physical quantity that characterizes the amount of heat required to change a substance’s temperature by a given amount. Heat capacity, ratio of heat absorbed by a material to the. Heat Capacity Definition Joules.
From www.slideserve.com
PPT Unit 10 (Chapter 17) Thermodynamics PowerPoint Presentation Heat Capacity Definition Joules C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It is measured in joules per kelvin and given by. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently,. Heat Capacity Definition Joules.
From slideplayer.com
Chapter 16 Reaction Energy ppt download Heat Capacity Definition Joules It is measured in joules per kelvin and given by. The temperature change (∆t) in the. A substance with a small heat. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. The unit of specific heat is joules per gram per degree celsius or j/g∙ ∘ c. The specific heat capacity of water. Heat Capacity Definition Joules.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download Heat Capacity Definition Joules This means that it takes 4,200 j to raise the. A substance with a small heat. Another unit of specific heat is calories per gram per degree celsius or j/cal∙ ∘ c. In si units, specific heat capacity (symbol: The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it. Heat Capacity Definition Joules.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types Heat Capacity Definition Joules The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The unit of specific heat is joules. Heat Capacity Definition Joules.
From haipernews.com
How To Calculate Heat Capacity Of Joules Haiper Heat Capacity Definition Joules In si units, specific heat capacity (symbol: The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius (or equivalently, 1 kelvin) c = q δt. It may also be expressed as j/kg·k. A substance with a small heat. The specific. Heat Capacity Definition Joules.