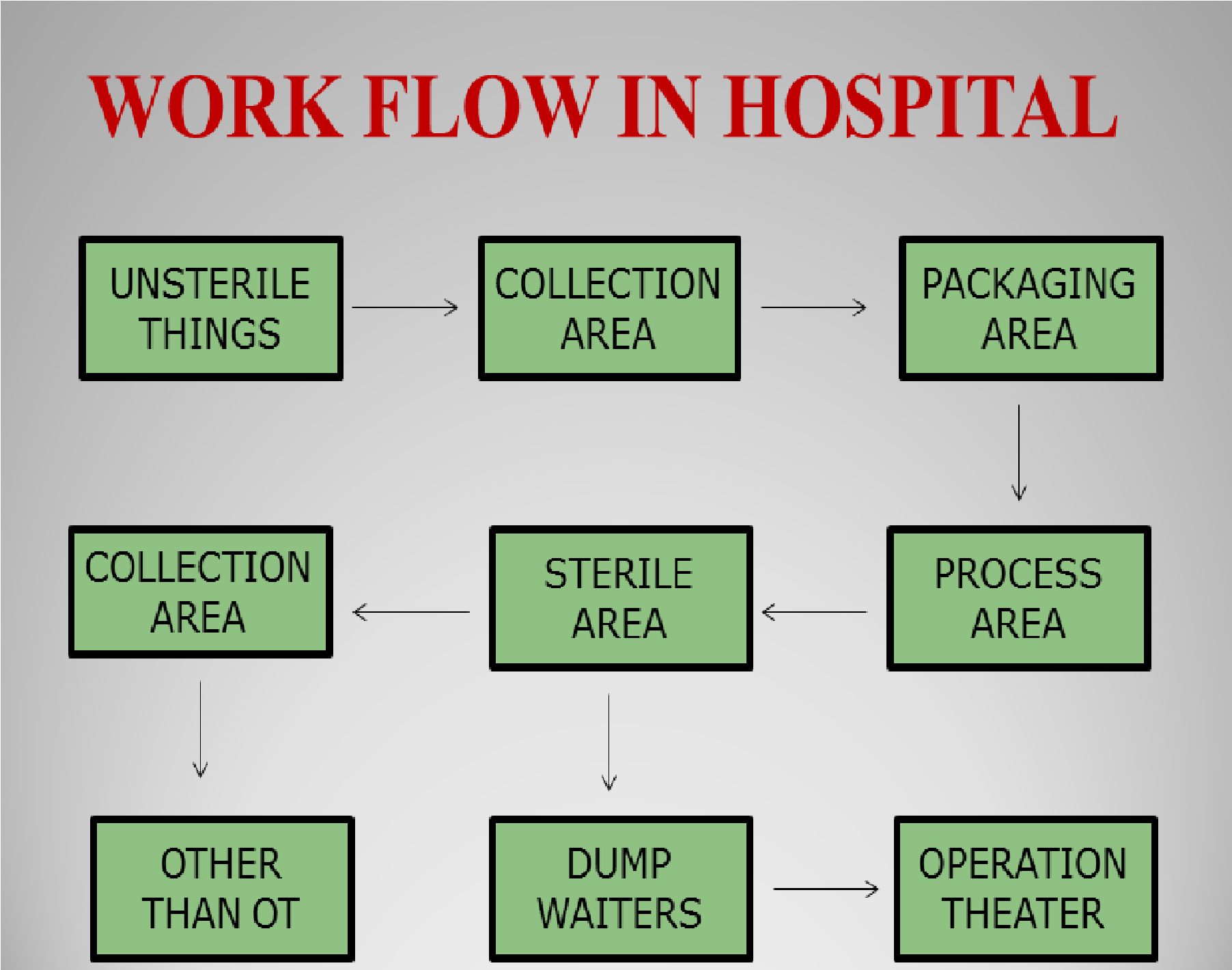
Which Sterilization Process Needs To Be In A Well Ventilated Area . Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. It should be performed just after. The central processing area (s) ideally should be divided into at least three areas:
from www.healthtard.com
It should be performed just after. The central processing area (s) ideally should be divided into at least three areas: Sterilization is the process that eliminates all living microorganisms including spores from surfaces. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time.
Sterilization in Hospitals
Which Sterilization Process Needs To Be In A Well Ventilated Area Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. The central processing area (s) ideally should be divided into at least three areas: It should be performed just after. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks.
From www.dentalclinic-knet.com
Dental Sterilization A StepbyStep Process Dental Clinic Which Sterilization Process Needs To Be In A Well Ventilated Area It should be performed just after. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. The central processing area (s) ideally should be divided into at least three areas: 1.1 the production of sterile preparations should be carried. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.vrogue.co
1kg Of Cleantissue For Sterilisation Of Explant In Pl vrogue.co Which Sterilization Process Needs To Be In A Well Ventilated Area It should be performed just after. The central processing area (s) ideally should be divided into at least three areas: Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. 1.1. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.cdc.gov
Improving Ventilation in Your Home CDC Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. It should be performed just after. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. 1.1 the production of sterile preparations. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From jewelprecision.com
What Is The Autoclave Process? Learn Here! Which Sterilization Process Needs To Be In A Well Ventilated Area It should be performed just after. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. When selecting a disinfectant for reprocessing medical equipment/devices in the. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From blog.medibuddy.in
6 Reasons why it is important to be in a ventilated room Which Sterilization Process Needs To Be In A Well Ventilated Area 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. When. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From slidetodoc.com
STERILIZATION Definitions Sterilization Process by which an article Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. The central processing area (s) ideally should be divided into at least three areas: Ensure cleaned ventilator is stored in an area where there is low risk of contamination between. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.crownasia.com.ph
Ideas on Passive Cooling in Your Home Crown Asia Which Sterilization Process Needs To Be In A Well Ventilated Area 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. The central processing area (s). Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.wikihow.com
3 Easy Ways to Set Up a Ventilated Area wikiHow Which Sterilization Process Needs To Be In A Well Ventilated Area Sterilization is the process that eliminates all living microorganisms including spores from surfaces. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. The central processing area (s) ideally should be divided into. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.rtix.com
BioCleanse® Tissue Sterilization Process RTI Surgical Which Sterilization Process Needs To Be In A Well Ventilated Area It should be performed just after. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. 1.1 the production of sterile preparations. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From microbeonline.com
Sterilization and Disinfection Methods • Microbe Online Which Sterilization Process Needs To Be In A Well Ventilated Area The central processing area (s) ideally should be divided into at least three areas: Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. When selecting a disinfectant for reprocessing medical. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From pharmacyscope.com
Physical Methods of Sterilization Pharmacy Scope Which Sterilization Process Needs To Be In A Well Ventilated Area The central processing area (s) ideally should be divided into at least three areas: 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Sterilization is the process that eliminates all living microorganisms. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From solutionpharmacy.in
Physical Methods of Sterilization Solution Parmacy Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From kerone.com
Different Types of Sterilization Process Which Sterilization Process Needs To Be In A Well Ventilated Area Sterilization is the process that eliminates all living microorganisms including spores from surfaces. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. •ensure. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From mungfali.com
Steam Sterilization Process Which Sterilization Process Needs To Be In A Well Ventilated Area The central processing area (s) ideally should be divided into at least three areas: Sterilization is the process that eliminates all living microorganisms including spores from surfaces. It should be performed just after. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. When selecting a disinfectant for reprocessing medical. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.bank2home.com
The Steam Sterilization Process Which Sterilization Process Needs To Be In A Well Ventilated Area 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. The central processing area (s) ideally should be divided into at least three areas: When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. •ensure the sterile storage area is a well‐ventilated area. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.medicaldesignandoutsourcing.com
A look at the industrial sterilization market Medical Design and Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. It should be performed just after. Sterilization is the process that eliminates all living microorganisms including spores. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.wikihow.com
3 Easy Ways to Set Up a Ventilated Area wikiHow Which Sterilization Process Needs To Be In A Well Ventilated Area It should be performed just after. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. •ensure the sterile storage area is a well‐ventilated. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.youtube.com
Understanding Steam Sterilization YouTube Which Sterilization Process Needs To Be In A Well Ventilated Area •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. The central processing. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From mungfali.com
Steam Sterilization Process Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. It should be performed just after. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. Ensure cleaned ventilator is stored in. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.healthtard.com
Sterilization in Hospitals Which Sterilization Process Needs To Be In A Well Ventilated Area The central processing area (s) ideally should be divided into at least three areas: •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Sterilization is the process that eliminates all living. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.slideserve.com
PPT STERILIZATION METHODS PowerPoint Presentation, free download ID Which Sterilization Process Needs To Be In A Well Ventilated Area Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. It should be performed just after. When selecting a disinfectant for reprocessing medical equipment/devices. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.sepsservices.com
5 Common Methods of Lab Sterilization SEPS Services Which Sterilization Process Needs To Be In A Well Ventilated Area The central processing area (s) ideally should be divided into at least three areas: It should be performed just after. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust,. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.cleanwastesystems.com
Sterilization Process Clean Waste Systems Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From microbenotes.com
Physical methods of sterilization Heat, Filtration, Radiation Which Sterilization Process Needs To Be In A Well Ventilated Area 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. It should be performed just after. The central processing area (s) ideally should be divided into at least three areas: When selecting. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From mungfali.com
Steam Sterilization Process Which Sterilization Process Needs To Be In A Well Ventilated Area Sterilization is the process that eliminates all living microorganisms including spores from surfaces. The central processing area (s) ideally should be divided into at least three areas: When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From microbeonline.com
Moist heat sterilization Definition, Principle, Advantages and Which Sterilization Process Needs To Be In A Well Ventilated Area 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. It should be performed just after. When selecting a disinfectant for reprocessing medical equipment/devices. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.self-build.co.uk
Home Ventilation Creating a Well Ventilated and Healthy House Build It Which Sterilization Process Needs To Be In A Well Ventilated Area Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. It should be performed just after. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. 1.1 the production of sterile preparations should be carried out. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From rbrlifescience.com
Heat Sterilization Method RBR Life Science Which Sterilization Process Needs To Be In A Well Ventilated Area Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. Sterilization is the process that eliminates all living microorganisms including spores from surfaces. The central processing area. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From bhsi.com.pk
ETO Commercial Sterilization B & H Surgical Instrument Which Sterilization Process Needs To Be In A Well Ventilated Area The central processing area (s) ideally should be divided into at least three areas: It should be performed just after. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From slidetodoc.com
Industrial Biotechnology Lecture 2 STERILIZATION Sterilization we will Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.compliancesigns.com
Vertical Use Chemicals In A Well Ventilated Area Sign ANSI Warning Which Sterilization Process Needs To Be In A Well Ventilated Area Sterilization is the process that eliminates all living microorganisms including spores from surfaces. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. 1.1 the production of. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.rsd-engineering.com
Ethylene oxide sterilization Process RSD Industrial sterilization Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. It should be performed just after. The central processing area (s) ideally should be divided into at least three areas: •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Ensure cleaned ventilator is stored. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.mdpi.com
Applied Sciences Free FullText Advances, Applications, and Which Sterilization Process Needs To Be In A Well Ventilated Area Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. 1.1 the production of sterile preparations should be carried out in clean areas, entry to which should be through airlocks. It should be performed just after. The central processing area (s) ideally should be. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.youtube.com
Sterilization techniques in tissue culture. YouTube Which Sterilization Process Needs To Be In A Well Ventilated Area When selecting a disinfectant for reprocessing medical equipment/devices in the health care setting, consideration needs to be. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact. Which Sterilization Process Needs To Be In A Well Ventilated Area.
From www.slideserve.com
PPT Sterilization & Disinfection PowerPoint Presentation, free Which Sterilization Process Needs To Be In A Well Ventilated Area Sterilization is the process that eliminates all living microorganisms including spores from surfaces. •ensure the sterile storage area is a well‐ventilated area that provides protection against dust, moisture, and temperature and. Ensure cleaned ventilator is stored in an area where there is low risk of contamination between uses, and that at least 1 minute of contact time. The central processing. Which Sterilization Process Needs To Be In A Well Ventilated Area.