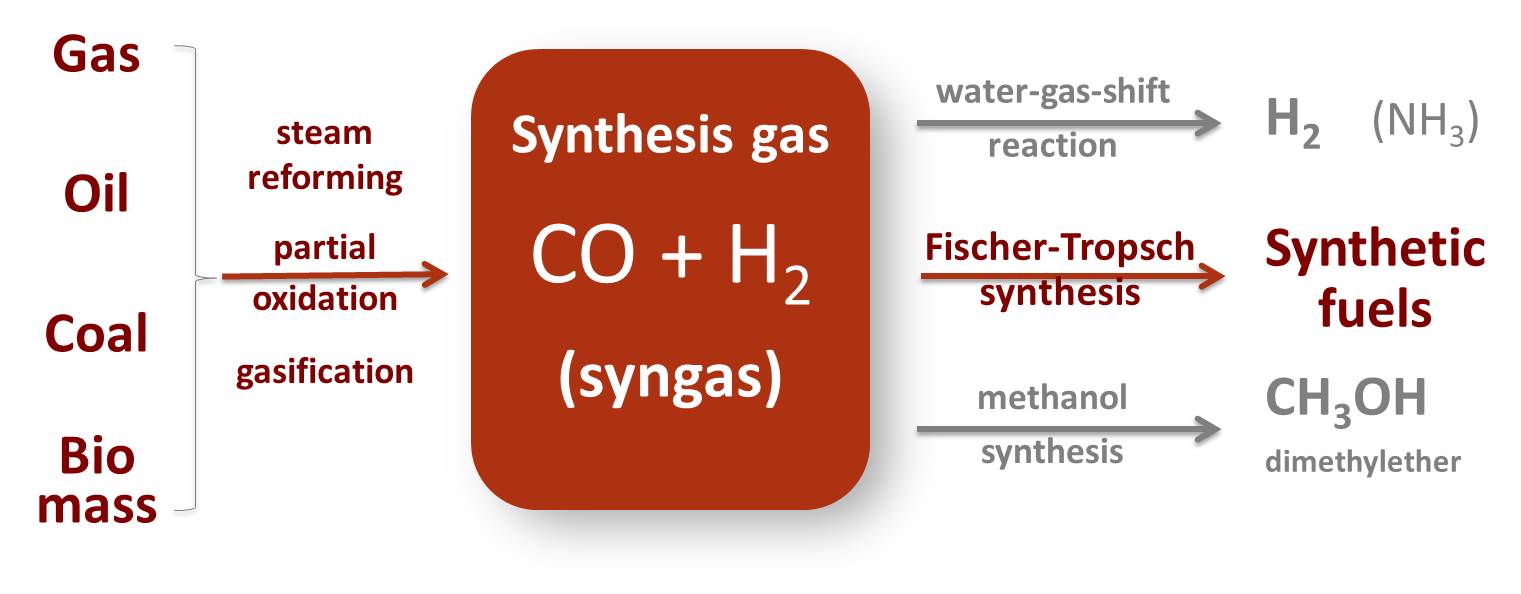
Fuel Chemical Formula . Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. To produce various grades, there is a blending of many refinery components, each of which. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Most of these compounds have. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal.
from www.syngaschem.com
To produce various grades, there is a blending of many refinery components, each of which. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Most of these compounds have. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds.
Synthesis Gas Chemistry and Synthetic Fuels Syngaschem BV
Fuel Chemical Formula Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Most of these compounds have. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. To produce various grades, there is a blending of many refinery components, each of which.
From www.researchgate.net
Chemical composition and activation energy for various JetA/JP8 fuels Fuel Chemical Formula Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that. Fuel Chemical Formula.
From biodieselisgood.org
Chemistry » Biodiesel is Good Fuel Chemical Formula Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Conventional gasoline is mostly a blended mixture of more. Fuel Chemical Formula.
From www.compoundchem.com
The Chemistry of Petrol & The Tetraethyl Lead Story Compound Interest Fuel Chemical Formula Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Most of these compounds have. To produce various grades, there is a blending of. Fuel Chemical Formula.
From www.e-education.psu.edu
Natural Gas Liquids FSC 432 Petroleum Refining Fuel Chemical Formula Most of these compounds have. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Gasoline, mixture of volatile, flammable. Fuel Chemical Formula.
From www.dreamstime.com
Fuel Molecules set stock vector. Illustration of decane 76462009 Fuel Chemical Formula Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Most of these compounds have. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. To produce various grades, there. Fuel Chemical Formula.
From www.syngaschem.com
Synthesis Gas Chemistry and Synthetic Fuels Syngaschem BV Fuel Chemical Formula Most of these compounds have. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Gasoline, mixture of volatile,. Fuel Chemical Formula.
From aldrabadacqoworkshopfix.z14.web.core.windows.net
R407c Refrigerant Chemical Formula Fuel Chemical Formula Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Most of these compounds have. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Hydrocarbons are the principal. Fuel Chemical Formula.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Chemical Formula Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels,. Fuel Chemical Formula.
From www.dreamstime.com
Gasoline stock illustration. Illustration of alkane, gasoline 83616024 Fuel Chemical Formula To produce various grades, there is a blending of many refinery components, each of which. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Most of these compounds have. Hydrocarbons are the principal constituents of petroleum and natural gas and. Fuel Chemical Formula.
From www.google.com
EP2342310A2 Jet fuel compositions Google Patents Fuel Chemical Formula Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing. Fuel Chemical Formula.
From chem.libretexts.org
15.7 Natural Gas and Petroleum Chemistry LibreTexts Fuel Chemical Formula Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants,. Fuel Chemical Formula.
From www.slideshare.net
Organic chemistry fossil fuels Fuel Chemical Formula Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing. Fuel Chemical Formula.
From www.youtube.com
Fossil Fuels IB Physics YouTube Fuel Chemical Formula To produce various grades, there is a blending of many refinery components, each of which. Most of these compounds have. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Combustion is a reaction between a hydrocarbon fuel (e.g., coal,. Fuel Chemical Formula.
From www.teachoo.com
What is Fuel? (with Examples) Chapter 6 Class 8 Science Notes Fuel Chemical Formula Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. To produce various grades, there is a blending of many refinery components, each of which. Most of these compounds have. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and. Fuel Chemical Formula.
From guide.alibaba.com
Cheap Fuel Chemical Formula, find Fuel Chemical Formula deals on line Fuel Chemical Formula Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. To produce various grades, there is a blending of many refinery components, each of which. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Gasoline is mixture of. Fuel Chemical Formula.
From chem.libretexts.org
2.2. Hydrogen, The Simplest Atom Chemistry LibreTexts Fuel Chemical Formula To produce various grades, there is a blending of many refinery components, each of which. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release. Fuel Chemical Formula.
From www.researchgate.net
Physical and chemical properties of the residual fuel oil Download Table Fuel Chemical Formula Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Hydrocarbons are the principal constituents of petroleum and natural. Fuel Chemical Formula.
From encyclopedia.pub
EFuels Encyclopedia MDPI Fuel Chemical Formula Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. To produce various grades, there is a blending of many refinery components, each of which. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Gasoline, mixture of volatile, flammable liquid. Fuel Chemical Formula.
From www.mdpi.com
Molecules Free FullText Catalytic Production of Jet Fuels from Biomass Fuel Chemical Formula Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing. Fuel Chemical Formula.
From www.mdpi.com
Applied Sciences Free FullText Using Canola Oil Biodiesel as an Fuel Chemical Formula Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Most of these compounds have. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. To produce various grades, there. Fuel Chemical Formula.
From www.mdpi.com
Molecules Free FullText Catalytic Production of Jet Fuels from Biomass Fuel Chemical Formula To produce various grades, there is a blending of many refinery components, each of which. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Most of these compounds have. Chemical fuels are substances that release energy by reacting. Fuel Chemical Formula.
From www.icao.int
Components of conventional jet fuel Fuel Chemical Formula Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Most of these compounds have. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process. Fuel Chemical Formula.
From www.revisechemistry.uk
Interpreting and interacting with Earth systems OCR Gateway C6 Fuel Chemical Formula Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. To produce various grades, there is a blending of many refinery components, each of which. Most of these. Fuel Chemical Formula.
From zzamfdyauo.blogspot.com
E10 Fuel Chemical Formula Ethanol Blended Petroleum Fuels Fuel Chemical Formula Most of these compounds have. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Hydrocarbons are the principal constituents. Fuel Chemical Formula.
From chem.libretexts.org
4.7 Fossil Fuels Chemistry LibreTexts Fuel Chemical Formula Most of these compounds have. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. To produce various grades, there is a blending of many refinery components, each of which. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by reacting with substances around them, most. Fuel Chemical Formula.
From www.dreamstime.com
Propane. Liquefied Petroleum Gas. Structural Chemical Formula and Fuel Chemical Formula Most of these compounds have. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Chemical fuels are substances that. Fuel Chemical Formula.
From www.researchgate.net
Physical and Chemical Properties of the Diesel Fuels Used Download Table Fuel Chemical Formula Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. To produce various grades, there is a blending of many refinery components, each of. Fuel Chemical Formula.
From favpng.com
Gasoline Molecule Chemical Substance Diesel Fuel Chemical Formula, PNG Fuel Chemical Formula Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Most of these compounds have. Gasoline, mixture of volatile, flammable. Fuel Chemical Formula.
From sciencetallis.weebly.com
7. Organic Chemistry THOMAS TALLIS SCIENCE Fuel Chemical Formula Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Most of these. Fuel Chemical Formula.
From nanohub.org
Resources Aviation Fuels Overview Watch Presentation Fuel Chemical Formula Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Most. Fuel Chemical Formula.
From sciencenotes.org
Fossil Fuel Examples and Uses Fuel Chemical Formula Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Most of these compounds have. Gasoline is mixture of different alkanes, alkenes and cycloalkanes compounds. Chemical fuels are substances that release energy by. Fuel Chemical Formula.
From www.thesciencehive.co.uk
Fuel Cells (AQA) — the science sauce Fuel Chemical Formula Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Most of these compounds have. To produce various grades, there is a blending of many refinery components, each. Fuel Chemical Formula.
From www.researchgate.net
Physical and chemical properties of fuel Download Table Fuel Chemical Formula Gasoline, mixture of volatile, flammable liquid hydrocarbons derived from petroleum and used as fuel for internal. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2),. Fuel Chemical Formula.
From www.nagwa.com
Question Video Identifying Which Equation Shows the Reaction at a Fuel Chemical Formula Hydrocarbons are the principal constituents of petroleum and natural gas and serve as fuels, lubricants, and raw materials for various products. Combustion is a reaction between a hydrocarbon fuel (e.g., coal, propane, wood, methane) and molecular oxygen (o 2), producing carbon dioxide (co 2), water (h 2 o), and heat. Conventional gasoline is mostly a blended mixture of more than. Fuel Chemical Formula.
From chemnotcheem.com
The chemistry of rocket science O Level Chemistry Stories Fuel Chemical Formula Chemical fuels are substances that release energy by reacting with substances around them, most notably by the process of combustion. Conventional gasoline is mostly a blended mixture of more than 200 different hydrocarbon liquids ranging from those containing 4 carbon atoms. To produce various grades, there is a blending of many refinery components, each of which. Hydrocarbons are the principal. Fuel Chemical Formula.