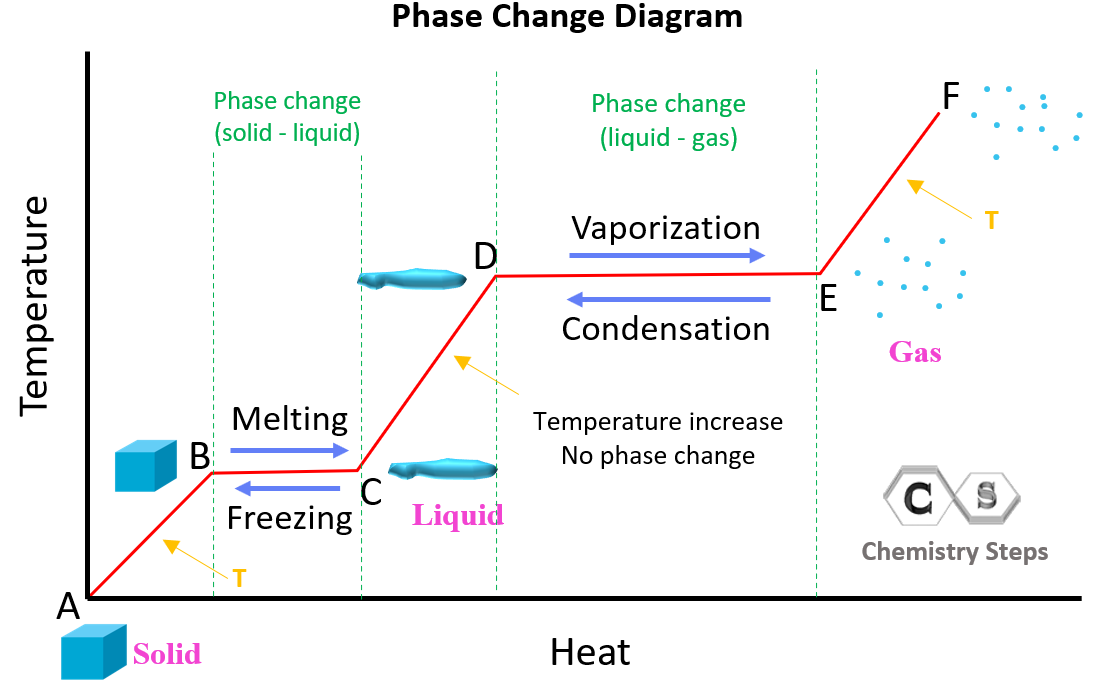
Temperature Phase Change Diagram . Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Solve calorimetry problems involving phase changes. The ice starts out at −20 °c °c ,. Every element and substance can transition from one phase to another at a. Phase transitions play an important theoretical and practical role in the study of heat flow. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Use phase diagrams to identify stable phases at given temperatures and. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to. In melting (or “ fusion ”), a solid turns into. Explain the construction and use of a typical phase diagram; A typical phase diagram has.
from general.chemistrysteps.com
Phase changes among the various phases of matter depend on temperature and pressure. Use phase diagrams to identify stable phases at given temperatures and. Phase transitions play an important theoretical and practical role in the study of heat flow. Explain the construction and use of a typical phase diagram; Every element and substance can transition from one phase to another at a. Solve calorimetry problems involving phase changes. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. In melting (or “ fusion ”), a solid turns into. The ice starts out at −20 °c °c ,.
Heat and Phase Change Diagrams Chemistry Steps
Temperature Phase Change Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase changes among the various phases of matter depend on temperature and pressure. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Explain the construction and use of a typical phase diagram; In melting (or “ fusion ”), a solid turns into. Solve calorimetry problems involving phase changes. A typical phase diagram has. Every element and substance can transition from one phase to another at a. Phase transitions play an important theoretical and practical role in the study of heat flow. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. The existence of the three phases with respect to. The ice starts out at −20 °c °c ,. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Use phase diagrams to identify stable phases at given temperatures and.
From general.chemistrysteps.com
Heat and Phase Change Diagrams Chemistry Steps Temperature Phase Change Diagram Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase changes among the various phases of matter depend on temperature and pressure. Explain the construction and use of a typical phase diagram; The existence of the three phases with respect to. Phase transition is when a substance changes from. Temperature Phase Change Diagram.
From hxepqhxji.blob.core.windows.net
Fuel Line Freeze Up at Norma Friddle blog Temperature Phase Change Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Solve calorimetry problems involving phase changes. The existence of the three phases with respect to. In melting (or “ fusion ”), a solid turns into. Phase diagram is a graphical representation of the physical states of a substance under different conditions of. Temperature Phase Change Diagram.
From serc.carleton.edu
Phase Rule Temperature Phase Change Diagram Phase transitions play an important theoretical and practical role in the study of heat flow. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Explain the construction and use. Temperature Phase Change Diagram.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Temperature Phase Change Diagram The ice starts out at −20 °c °c ,. The existence of the three phases with respect to. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase changes among the various phases of matter depend on temperature and pressure. Phase transition is when a substance changes from a. Temperature Phase Change Diagram.
From www.worksheeto.com
Physics Worksheet Category Page 1 Temperature Phase Change Diagram Use phase diagrams to identify stable phases at given temperatures and. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Explain the construction and use of a typical phase diagram; Solve calorimetry problems involving phase changes. Every element and substance can transition from one phase to another at a. A phase. Temperature Phase Change Diagram.
From www.vrogue.co
Solved Part Phase Diagrams Shown Below Is A Phase Dia vrogue.co Temperature Phase Change Diagram Phase transitions play an important theoretical and practical role in the study of heat flow. A typical phase diagram has. Every element and substance can transition from one phase to another at a. Solve calorimetry problems involving phase changes. Use phase diagrams to identify stable phases at given temperatures and. Phase transition is when a substance changes from a solid,. Temperature Phase Change Diagram.
From quizzschoolconnor123.z19.web.core.windows.net
Show The Phase Change Diagram Temperature Phase Change Diagram In melting (or “ fusion ”), a solid turns into. The ice starts out at −20 °c °c ,. Solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. Use phase diagrams to identify stable phases at given temperatures and. Phase transition is when a substance changes from a. Temperature Phase Change Diagram.
From gioubljqa.blob.core.windows.net
Solid Liquid Gas Diagram Graph at Charles Hunt blog Temperature Phase Change Diagram Phase transitions play an important theoretical and practical role in the study of heat flow. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Explain the construction and use of a typical phase diagram; Every element and substance can transition from one phase to another at a. In melting (or “. Temperature Phase Change Diagram.
From www.101diagrams.com
Phase Change Diagrams 101 Diagrams Temperature Phase Change Diagram Explain the construction and use of a typical phase diagram; A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase transition is when a substance changes from a solid, liquid, or. Temperature Phase Change Diagram.
From sciencenotes.org
States of Matter Temperature Phase Change Diagram A typical phase diagram has. Every element and substance can transition from one phase to another at a. In melting (or “ fusion ”), a solid turns into. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. A phase diagram indicating the temperature changes of water as energy is added is. Temperature Phase Change Diagram.
From www.jove.com
Equation1 Temperature Phase Change Diagram In melting (or “ fusion ”), a solid turns into. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Explain the construction and use of a typical phase diagram; The existence of the three phases with respect to. Phase changes among the various phases of matter depend on temperature and pressure.. Temperature Phase Change Diagram.
From circuitmemogttyf.z21.web.core.windows.net
Phase Diagram For Circuits Temperature Phase Change Diagram In melting (or “ fusion ”), a solid turns into. A typical phase diagram has. The ice starts out at −20 °c °c ,. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Explain the construction and use of a typical phase diagram; Phase transitions play an important theoretical. Temperature Phase Change Diagram.
From link.springer.com
Aluminum Titanate and HighTemperature HeatResistant Compositions with Temperature Phase Change Diagram The ice starts out at −20 °c °c ,. Solve calorimetry problems involving phase changes. Phase changes among the various phases of matter depend on temperature and pressure. Every element and substance can transition from one phase to another at a. A typical phase diagram has. Use phase diagrams to identify stable phases at given temperatures and. Phase transition is. Temperature Phase Change Diagram.
From lessonlibrarymildew.z21.web.core.windows.net
Diagram Of Phase Changes Temperature Phase Change Diagram A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase transitions play an important theoretical and practical role in the study of heat flow. The ice starts out at −20 °c. Temperature Phase Change Diagram.
From biusw6sguidediagram.z13.web.core.windows.net
Generator Changeover Switch Wiring Diagram Queensland Temperature Phase Change Diagram A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. A typical phase diagram has. The ice starts out at −20 °c °c ,. Phase changes among the various phases of matter depend on temperature and pressure. In melting (or “ fusion ”), a solid turns into. Solve calorimetry problems involving phase. Temperature Phase Change Diagram.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Temperature Phase Change Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. The ice starts out. Temperature Phase Change Diagram.
From ar.inspiredpencil.com
Sublimation Phase Diagram Temperature Phase Change Diagram Phase changes among the various phases of matter depend on temperature and pressure. In melting (or “ fusion ”), a solid turns into. Explain the construction and use of a typical phase diagram; Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Use phase diagrams to identify stable phases. Temperature Phase Change Diagram.
From schematicmoveable.z14.web.core.windows.net
Phase Diagrams Understanding The Basics Temperature Phase Change Diagram Solve calorimetry problems involving phase changes. Phase transitions play an important theoretical and practical role in the study of heat flow. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. The. Temperature Phase Change Diagram.
From pressbooks.bccampus.ca
Phase Changes Basic HVAC Temperature Phase Change Diagram Solve calorimetry problems involving phase changes. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The existence of the three phases with respect to. Phase transitions play an important theoretical and practical role in the study of heat flow. A phase diagram indicating the temperature changes of water as energy is. Temperature Phase Change Diagram.
From circuitmemogttyf.z21.web.core.windows.net
Phase Diagram For Circuits Temperature Phase Change Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Explain the construction and use of a typical phase diagram; The ice starts out at −20 °c °c ,. Every element and substance can transition from one phase to another at a. The existence of the three phases with respect to. Use. Temperature Phase Change Diagram.
From monroe.com.au
PDF) Phase diagram calculation of molten salt hydrates using the Temperature Phase Change Diagram Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase transitions play an important theoretical and practical role in the study of heat flow. The ice starts out at −20 °c °c ,. The existence of the three phases with respect to. Explain the construction and use of a. Temperature Phase Change Diagram.
From link.springer.com
Aluminum Titanate and HighTemperature HeatResistant Compositions with Temperature Phase Change Diagram Explain the construction and use of a typical phase diagram; Every element and substance can transition from one phase to another at a. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase changes among the various phases of matter depend on temperature and pressure. A phase diagram indicating. Temperature Phase Change Diagram.
From socratic.org
What happens to the temperature during a phase change? Socratic Temperature Phase Change Diagram Solve calorimetry problems involving phase changes. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Explain the construction and use of a typical phase diagram; Phase transitions play an important theoretical and practical role in the study of heat flow. Every element and substance can transition from one phase to another. Temperature Phase Change Diagram.
From schematicmoveable.z14.web.core.windows.net
Reading A Phase Diagram Temperature Phase Change Diagram Explain the construction and use of a typical phase diagram; Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The ice starts out at −20 °c °c ,. In melting (or “ fusion ”), a solid turns into. Phase changes among the various phases of matter depend on temperature and pressure.. Temperature Phase Change Diagram.
From www.reddit.com
DIY water filtration inspiration (water should be boiled after its Temperature Phase Change Diagram Solve calorimetry problems involving phase changes. In melting (or “ fusion ”), a solid turns into. Every element and substance can transition from one phase to another at a. The existence of the three phases with respect to. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The ice starts out. Temperature Phase Change Diagram.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Temperature Phase Change Diagram A typical phase diagram has. Phase transitions play an important theoretical and practical role in the study of heat flow. The ice starts out at −20 °c °c ,. Explain the construction and use of a typical phase diagram; Use phase diagrams to identify stable phases at given temperatures and. Phase diagram is a graphical representation of the physical states. Temperature Phase Change Diagram.
From www.chegg.com
Solved The figure below is the pressuretemperature phase Temperature Phase Change Diagram Use phase diagrams to identify stable phases at given temperatures and. Solve calorimetry problems involving phase changes. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The existence of the three phases with respect to. Every element and substance can transition from one phase to another at a. A typical phase. Temperature Phase Change Diagram.
From www.savemyexams.com
Energy of Phase Changes College Board AP® Chemistry Study Guides 2022 Temperature Phase Change Diagram Solve calorimetry problems involving phase changes. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Phase transition is when a substance changes from a solid, liquid, or gas state. Temperature Phase Change Diagram.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Temperature Phase Change Diagram Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. The existence of the three phases with respect to. A typical phase diagram has. Phase transitions play an important theoretical and practical role in the study of heat flow. Phase changes among the various phases of matter depend on temperature. Temperature Phase Change Diagram.
From conceptgroupllc.com
What is phase change? Explained by Thermal Engineers Temperature Phase Change Diagram A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Use phase diagrams to identify stable phases at given temperatures and. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. The ice starts out at −20 °c °c ,. The existence of the. Temperature Phase Change Diagram.
From www.expii.com
Phase Change Diagrams — Overview & Examples Expii Temperature Phase Change Diagram A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Phase changes among the various phases of matter depend on temperature and pressure. The existence of the three phases with respect to. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. A typical. Temperature Phase Change Diagram.
From printablezonebardot.z21.web.core.windows.net
Phase Diagram Examples Temperature Phase Change Diagram A typical phase diagram has. Use phase diagrams to identify stable phases at given temperatures and. Phase transitions play an important theoretical and practical role in the study of heat flow. Every element and substance can transition from one phase to another at a. Phase changes among the various phases of matter depend on temperature and pressure. A phase diagram. Temperature Phase Change Diagram.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Temperature Phase Change Diagram The existence of the three phases with respect to. In melting (or “ fusion ”), a solid turns into. Phase changes among the various phases of matter depend on temperature and pressure. A phase diagram indicating the temperature changes of water as energy is added is shown in figure 11.10. Explain the construction and use of a typical phase diagram;. Temperature Phase Change Diagram.
From printablezonebardot.z21.web.core.windows.net
Phase Diagram Examples Temperature Phase Change Diagram The ice starts out at −20 °c °c ,. Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. Phase transitions play an important theoretical and practical role in the study of. Temperature Phase Change Diagram.
From glossary.periodni.com
Critical point Chemistry Dictionary & Glossary Temperature Phase Change Diagram Phase transition is when a substance changes from a solid, liquid, or gas state to a different state. Explain the construction and use of a typical phase diagram; Phase transitions play an important theoretical and practical role in the study of heat flow. The existence of the three phases with respect to. Every element and substance can transition from one. Temperature Phase Change Diagram.