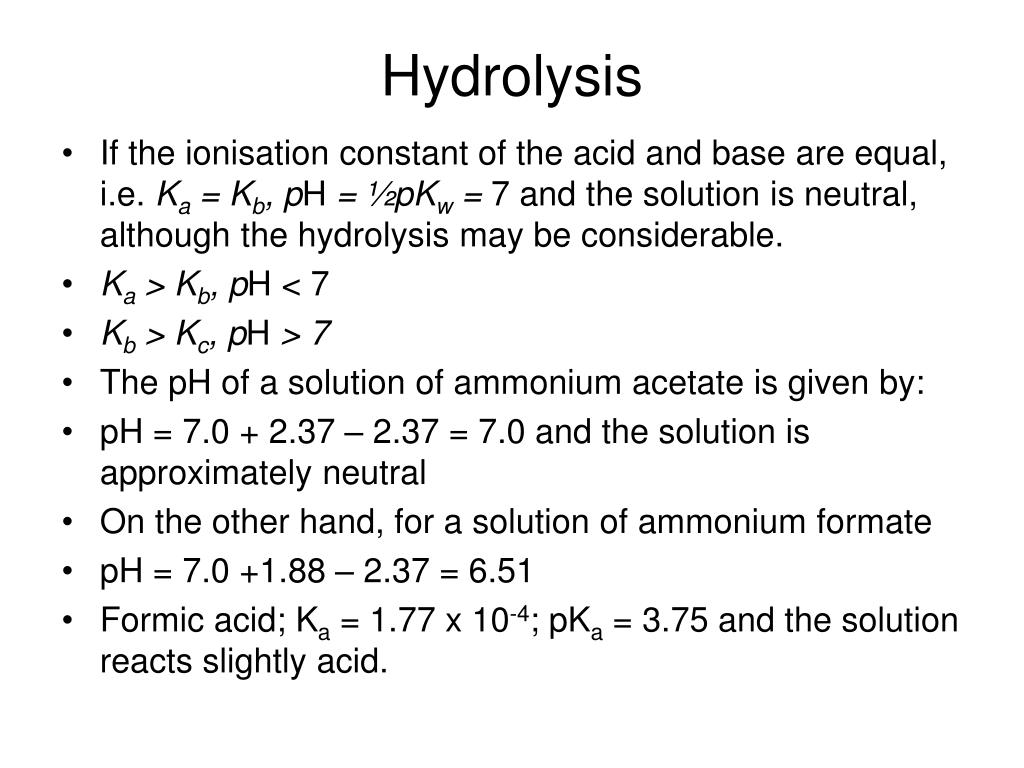
Base Hydrolysis Definition . And typically, water is used to break chemical bonds in the other. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. The reaction of water with another chemical compound results in the formation of two or more products. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. Calculate the ph of a salt solution. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Predict the acidity of a salt solution. Acidic hydrolysis of an ester gives a. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. The other reactants, and the products of hydrolysis,.
from www.slideserve.com
The other reactants, and the products of hydrolysis,. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. And typically, water is used to break chemical bonds in the other. Calculate the ph of a salt solution. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Acidic hydrolysis of an ester gives a. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. The reaction of water with another chemical compound results in the formation of two or more products.
PPT Acid Base Hydrolysis PowerPoint Presentation, free download ID6797412
Base Hydrolysis Definition Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The other reactants, and the products of hydrolysis,. Calculate the ph of a salt solution. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The reaction of water with another chemical compound results in the formation of two or more products. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Acidic hydrolysis of an ester gives a. And typically, water is used to break chemical bonds in the other. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Predict the acidity of a salt solution.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Base Hydrolysis Definition Calculate the ph of a salt solution. The other reactants, and the products of hydrolysis,. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. The reaction of water with another chemical compound results in the formation. Base Hydrolysis Definition.
From www.slideserve.com
PPT HYDROLYSIS PowerPoint Presentation, free download ID6574288 Base Hydrolysis Definition And typically, water is used to break chemical bonds in the other. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Predict the acidity of a salt solution. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic bonds and highly polar bonds can “hydrolyze,”. Base Hydrolysis Definition.
From www.masterorganicchemistry.com
Basic Hydrolysis of Esters Saponification Base Hydrolysis Definition Predict the acidity of a salt solution. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. The other reactants, and the products of hydrolysis,. The reaction of water with another chemical compound results in the formation of two or more products. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break.. Base Hydrolysis Definition.
From www.youtube.com
Chemistry Acids and Bases Hydrolysis of salts (Simplified) YouTube Base Hydrolysis Definition A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Predict the acidity of a salt solution. Hydrolysis is a type of decomposition reaction where one of the reactants is water; The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. And typically, water is used to. Base Hydrolysis Definition.
From www.slideserve.com
PPT Equilibrium in Solutions PowerPoint Presentation, free download ID3325525 Base Hydrolysis Definition A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Predict the acidity of a salt solution. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. And typically, water is used to break chemical bonds in the other. Hydrolysis is a type of decomposition reaction where one of the reactants. Base Hydrolysis Definition.
From www.youtube.com
Acids and Bases Grade 12 Hydrolysis YouTube Base Hydrolysis Definition Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. Calculate the ph of a salt solution. Predict the acidity of a salt solution. Acidic hydrolysis of an ester gives a. The reaction of water with another chemical compound results in the formation of two or more products. The definition of hydrolysis is the breaking of a chemical. Base Hydrolysis Definition.
From www.masterorganicchemistry.com
Amide Hydrolysis Using Acid Or Base Master Organic Chemistry Base Hydrolysis Definition Acidic hydrolysis of an ester gives a. The reaction of water with another chemical compound results in the formation of two or more products. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. And typically, water is used to break chemical bonds in the other. The definition of hydrolysis is the breaking of. Base Hydrolysis Definition.
From www.slideserve.com
PPT Acids & Bases Hydrolysis PowerPoint Presentation, free download ID6880951 Base Hydrolysis Definition Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. And typically, water is used to break chemical bonds in the other. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Acidic. Base Hydrolysis Definition.
From www.slideserve.com
PPT HYDROLYSIS REACTIONS PowerPoint Presentation, free download ID378930 Base Hydrolysis Definition Predict the acidity of a salt solution. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. Hydrolysis is a type of decomposition reaction where one of the reactants is water; A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Calculate the ph of a salt solution. And typically, water. Base Hydrolysis Definition.
From www.britannica.com
Hydrolysis Definition, Examples, & Facts Britannica Base Hydrolysis Definition Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The other reactants, and the products of hydrolysis,. Calculate the ph of a salt solution. The reaction of water with another chemical compound results in the formation of two or more products. Predict the acidity of a salt solution. And typically, water is used to break chemical bonds. Base Hydrolysis Definition.
From www.slideserve.com
PPT Salts in Solution PowerPoint Presentation, free download ID6881756 Base Hydrolysis Definition And typically, water is used to break chemical bonds in the other. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Acidic hydrolysis of an ester gives a. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Hydrolysis, in chemistry and physiology, a double decomposition reaction with water. Base Hydrolysis Definition.
From www.chemistrysteps.com
Amide Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Base Hydrolysis Definition The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Predict the acidity of a salt solution. The other reactants, and the products of hydrolysis,. The reaction of water with another chemical compound results in the formation of two or. Base Hydrolysis Definition.
From www.researchgate.net
Mechanisms of ester hydrolysis under acid or base catalysis. Download Scientific Diagram Base Hydrolysis Definition The other reactants, and the products of hydrolysis,. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. And typically, water is used to break chemical bonds in the other. Predict the acidity of a salt solution. Calculate the ph of a salt solution. The reaction of water with another chemical compound results in. Base Hydrolysis Definition.
From www.animalia-life.club
Simple Hydrolysis Reaction Base Hydrolysis Definition The other reactants, and the products of hydrolysis,. And typically, water is used to break chemical bonds in the other. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Calculate the ph of a salt solution. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. Hydrolysis, in chemistry and. Base Hydrolysis Definition.
From www.animalia-life.club
Simple Hydrolysis Reaction Base Hydrolysis Definition Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The reaction of water with another chemical compound results in the formation of two or more products. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules.. Base Hydrolysis Definition.
From www.youtube.com
Base hydrolysis of esters Real Chemistry YouTube Base Hydrolysis Definition The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. The other reactants, and the products of hydrolysis,. Predict the acidity of a salt solution. The reaction of water with another chemical compound results in the formation. Base Hydrolysis Definition.
From www.slideserve.com
PPT Substitution reactions at octahedral complexes acid and base hydrolysis PowerPoint Base Hydrolysis Definition Acidic hydrolysis of an ester gives a. Hydrolysis is a type of decomposition reaction where one of the reactants is water; The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Calculate the ph of a salt solution. The other reactants, and the products of hydrolysis,. And typically, water is used to break chemical. Base Hydrolysis Definition.
From www.slideserve.com
PPT Carboxylic acids and their derivatives PowerPoint Presentation, free download ID339482 Base Hydrolysis Definition Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. And typically, water is used to break chemical bonds in the other. Calculate the ph of a salt solution. Predict the acidity of a salt solution. Hydrolysis is a type of. Base Hydrolysis Definition.
From www.thoughtco.com
An Explanation of the Process Hydrolysis Base Hydrolysis Definition And typically, water is used to break chemical bonds in the other. Calculate the ph of a salt solution. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. The other reactants, and the products of hydrolysis,. A hydrolysis reaction. Base Hydrolysis Definition.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Base Hydrolysis Definition The other reactants, and the products of hydrolysis,. Predict the acidity of a salt solution. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Calculate the ph of a salt solution. Importantly, only ionic bonds and. Base Hydrolysis Definition.
From www.slideserve.com
PPT Acid Base Hydrolysis PowerPoint Presentation, free download ID6797412 Base Hydrolysis Definition Predict the acidity of a salt solution. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Acidic hydrolysis of an ester gives a. The reaction of water with another chemical compound results in the formation of two or more products. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one. Base Hydrolysis Definition.
From www.slideserve.com
PPT Acid Base Hydrolysis PowerPoint Presentation, free download ID6797412 Base Hydrolysis Definition Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Predict the acidity of a salt solution. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. The reaction of water with another chemical compound. Base Hydrolysis Definition.
From mungfali.com
Hydrolysis Reaction Mechanism Base Hydrolysis Definition Calculate the ph of a salt solution. Acidic hydrolysis of an ester gives a. The reaction of water with another chemical compound results in the formation of two or more products. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. A hydrolysis reaction. Base Hydrolysis Definition.
From www.chemistrysteps.com
Amide Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Base Hydrolysis Definition Acidic hydrolysis of an ester gives a. And typically, water is used to break chemical bonds in the other. The other reactants, and the products of hydrolysis,. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis. Base Hydrolysis Definition.
From www.slideserve.com
PPT Acid Base Hydrolysis PowerPoint Presentation, free download ID6797412 Base Hydrolysis Definition Hydrolysis is a type of decomposition reaction where one of the reactants is water; Calculate the ph of a salt solution. Predict the acidity of a salt solution. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller. Base Hydrolysis Definition.
From www.slideserve.com
PPT Acid Base Hydrolysis PowerPoint Presentation, free download ID379277 Base Hydrolysis Definition The other reactants, and the products of hydrolysis,. The reaction of water with another chemical compound results in the formation of two or more products. And typically, water is used to break chemical bonds in the other. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Acidic hydrolysis of an ester gives a.. Base Hydrolysis Definition.
From www.slideserve.com
PPT Substitution reactions at octahedral complexes acid and base hydrolysis PowerPoint Base Hydrolysis Definition The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Acidic hydrolysis of an ester gives a. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. Importantly, only ionic bonds and highly polar. Base Hydrolysis Definition.
From www.worksheetsplanet.com
What is Hydrolysis Definition of Hydrolysis Base Hydrolysis Definition Calculate the ph of a salt solution. Predict the acidity of a salt solution. Acidic hydrolysis of an ester gives a. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The other reactants, and the products of hydrolysis,. The reaction of water with another chemical compound results in the formation of two or more products. Hydrolysis is. Base Hydrolysis Definition.
From www.researchgate.net
(A) Neutral and (B) base hydrolysis of haloacetonitriles. (A)... Download Scientific Diagram Base Hydrolysis Definition Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. And typically, water is used to break chemical bonds in the other. Hydrolysis, in chemistry. Base Hydrolysis Definition.
From www.slideserve.com
PPT Acid Base Hydrolysis PowerPoint Presentation, free download ID6797412 Base Hydrolysis Definition Calculate the ph of a salt solution. And typically, water is used to break chemical bonds in the other. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The definition of hydrolysis is the breaking of a chemical bond through a reaction with water. The reaction of water with another chemical compound results in the formation of. Base Hydrolysis Definition.
From www.scribd.com
Hydrolysis Hydrolysis Salt (Chemistry) Base Hydrolysis Definition Calculate the ph of a salt solution. The other reactants, and the products of hydrolysis,. Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. And typically, water is used to break chemical bonds in the other. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic. Base Hydrolysis Definition.
From www.youtube.com
Acids and Bases Part 7 Hydrolysis YouTube Base Hydrolysis Definition The reaction of water with another chemical compound results in the formation of two or more products. And typically, water is used to break chemical bonds in the other. Hydrolysis is a type of decomposition reaction where one of the reactants is water; A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules.. Base Hydrolysis Definition.
From www.slideserve.com
PPT HYDROLYSIS REACTIONS PowerPoint Presentation, free download ID378930 Base Hydrolysis Definition Hydrolysis, in chemistry and physiology, a double decomposition reaction with water as one of the reactants. And typically, water is used to break chemical bonds in the other. Acidic hydrolysis of an ester gives a. The other reactants, and the products of hydrolysis,. Hydrolysis is a type of decomposition reaction where one of the reactants is water; Importantly, only ionic. Base Hydrolysis Definition.
From www.animalia-life.club
Simple Hydrolysis Reaction Base Hydrolysis Definition The other reactants, and the products of hydrolysis,. A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Acidic hydrolysis of an ester gives a. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. Predict the acidity of a salt solution. Hydrolysis is a type of decomposition reaction where one. Base Hydrolysis Definition.
From www.animalia-life.club
Simple Hydrolysis Reaction Base Hydrolysis Definition A hydrolysis reaction is a reaction in which one molecule breaks apart to form multiple smaller molecules. Acidic hydrolysis of an ester gives a. Predict the acidity of a salt solution. Importantly, only ionic bonds and highly polar bonds can “hydrolyze,” meaning break. The other reactants, and the products of hydrolysis,. Hydrolysis is a type of decomposition reaction where one. Base Hydrolysis Definition.