Chemistry Gas Laws Lab Answers . Complete all sections of this report and answer all questions in complete sentences for full credit. Experiment 11 the gas laws. Applying these laws to compare gases under two different sets of conditions gives the formulas: Answer questions in complete sentences and support with data from lab. Which gas law is this experiment investigating? P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. Complete all sections of this report and answer all questions in complete sentences for full credit. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2).
from www.chegg.com
Complete all sections of this report and answer all questions in complete sentences for full credit. Answer questions in complete sentences and support with data from lab. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Applying these laws to compare gases under two different sets of conditions gives the formulas: As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Experiment 11 the gas laws. Complete all sections of this report and answer all questions in complete sentences for full credit.
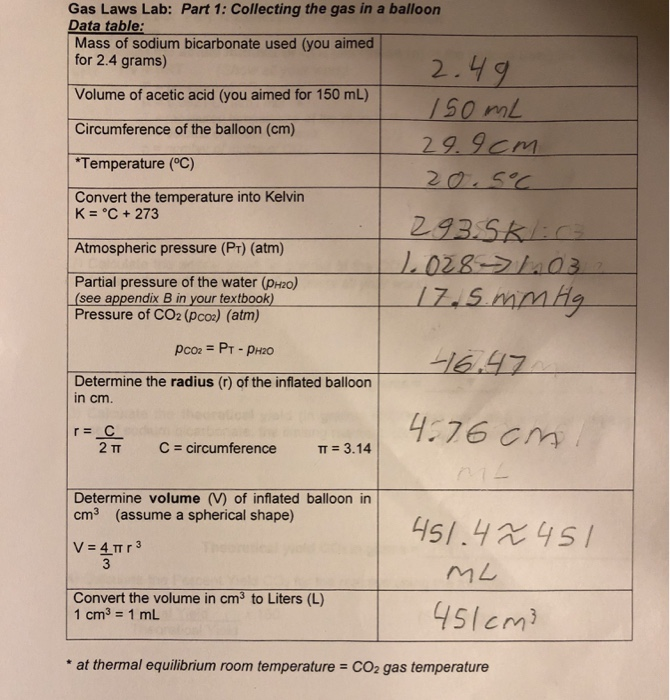
Gas Laws Lab Part 1 Collecting the gas in a balloon
Chemistry Gas Laws Lab Answers In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. Which gas law is this experiment investigating? In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Answer questions in complete sentences and support with data from lab. Experiment 11 the gas laws. As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Complete all sections of this report and answer all questions in complete sentences for full credit. Complete all sections of this report and answer all questions in complete sentences for full credit. Applying these laws to compare gases under two different sets of conditions gives the formulas:
From www.scribd.com
Chem Lab Report 9 (2)Gas Law Gases Temperature Chemistry Gas Laws Lab Answers In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Complete all sections of this report and answer all questions in complete sentences for full credit. Experiment 11 the gas laws. Answer questions in complete sentences and support with data from lab. A gas will act like an ideal gas if. Chemistry Gas Laws Lab Answers.
From mungfali.com
Exploring Gas Laws Lab Chemistry Gas Laws Lab Answers Complete all sections of this report and answer all questions in complete sentences for full credit. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Experiment 11 the gas laws. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved Chemistry Gas Laws Lab Answers Experiment 11 the gas laws. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Which gas law is this experiment investigating? Applying these laws to compare gases under two different sets of conditions gives the formulas: In this experiment you will (1) determine whether boyle’s. Chemistry Gas Laws Lab Answers.
From www.studypool.com
SOLUTION Lab 12 Gas Laws Studypool Chemistry Gas Laws Lab Answers Experiment 11 the gas laws. As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. Applying these laws to compare gases under two different sets of conditions gives the formulas: A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high.. Chemistry Gas Laws Lab Answers.
From www.studypool.com
SOLUTION Ideal gas law worksheet 2 answer Studypool Chemistry Gas Laws Lab Answers In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Experiment 11 the gas laws. Complete all sections of this report and answer all questions in complete sentences for full credit. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. A gas will act like an ideal gas if its gas molecules are small,. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved Gas Law Chem Lab (Two Questions) 1.) Through the Chemistry Gas Laws Lab Answers Applying these laws to compare gases under two different sets of conditions gives the formulas: Answer questions in complete sentences and support with data from lab. Experiment 11 the gas laws. Complete all sections of this report and answer all questions in complete sentences for full credit. A gas will act like an ideal gas if its gas molecules are. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved Name Gas Laws Lab instructor may ask you to answer Chemistry Gas Laws Lab Answers Experiment 11 the gas laws. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Complete all sections of this report and answer all questions in complete sentences for full credit. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Applying. Chemistry Gas Laws Lab Answers.
From obropolox.blogspot.com
40 chemistry gas laws worksheet with work Worksheet Resource Chemistry Gas Laws Lab Answers P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Experiment 11 the gas laws. As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. Complete all sections of this report and answer all questions in complete sentences for. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Gas Laws Lab Part 1 Collecting the gas in a balloon Chemistry Gas Laws Lab Answers Complete all sections of this report and answer all questions in complete sentences for full credit. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Which gas law is this experiment. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Gas Laws Lab Calculations Chem 112 Studocu Chemistry Gas Laws Lab Answers As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. Complete all sections of this report and answer all questions in complete sentences for full credit. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Answer questions in complete sentences and support with data. Chemistry Gas Laws Lab Answers.
From goodimg.co
️Gas Laws Worksheet Chemistry Answers Free Download Goodimg.co Chemistry Gas Laws Lab Answers In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Complete all sections of this report and answer all questions in complete sentences for full credit. Complete all sections of this report and answer all questions in complete sentences for full credit. As the bubbles rise, the pressure decreases, so their. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved CHEMISTRY. IDEAL GAS LAW CONSTANT INTRODUCTION Chemistry Gas Laws Lab Answers In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Experiment 11 the gas laws. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Answer questions in complete sentences and support with data from lab. As. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved CHEM 1211 Lab Manual Revised 052017 Ideal Gas Law, Chemistry Gas Laws Lab Answers In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Which gas law is this experiment investigating? Complete all sections of this report and answer all questions in complete sentences for full credit. Applying these laws to compare gases under two different sets of conditions gives the formulas: Complete all sections. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Experiment 8 Gas Laws Lab instructions Experiment 8 Gas Laws Required reading Ebbing Chemistry Gas Laws Lab Answers Which gas law is this experiment investigating? A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. As the bubbles rise,. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved CHEM 1211 Lab ManualRevised 05/2017 Ideal Gas Law, Chemistry Gas Laws Lab Answers P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. Answer questions in complete sentences and support with data from lab. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Complete all sections of this report and answer all questions in complete sentences for full credit. In this simulation,. Chemistry Gas Laws Lab Answers.
From animalia-life.club
Combined Gas Law Worksheet Answers Chemistry Gas Laws Lab Answers Answer questions in complete sentences and support with data from lab. Which gas law is this experiment investigating? As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. Applying these laws to compare gases under two different sets of conditions gives the formulas: Experiment 11 the gas laws. Complete all sections of this report. Chemistry Gas Laws Lab Answers.
From studylib.net
Chemistry Gas Laws ACT Questions [USE TO STUDY Chemistry Gas Laws Lab Answers Which gas law is this experiment investigating? Experiment 11 the gas laws. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. In this simulation, students will investigate three of the fundamental gas laws, including. Chemistry Gas Laws Lab Answers.
From crosweaphari.weebly.com
Ideal Gas Law Chem Worksheet 144 Answer Key LINK Chemistry Gas Laws Lab Answers Experiment 11 the gas laws. Complete all sections of this report and answer all questions in complete sentences for full credit. Complete all sections of this report and answer all questions in complete sentences for full credit. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Gas laws lab for chemistry (filled out) docu CHEM 095 Studocu Chemistry Gas Laws Lab Answers P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. Experiment 11 the gas laws. Applying these laws to compare gases under two different sets of conditions gives the formulas: A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Which gas law is this experiment investigating? In this simulation,. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Chem (Lab Sect 029) Experiment 7 Gas Laws Write Up 1 Experiment 7 Name Zoe Raphael Chemistry Gas Laws Lab Answers Complete all sections of this report and answer all questions in complete sentences for full credit. Complete all sections of this report and answer all questions in complete sentences for full credit. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Answer questions in complete sentences and support with data. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved Virtual Gas Law Simulation Lab Chem 1A_Lab Report Chemistry Gas Laws Lab Answers In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Answer questions in complete sentences and support with data from lab. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Which gas law is this experiment. Chemistry Gas Laws Lab Answers.
From materialcampusrosefish.z21.web.core.windows.net
Combined Gas Law Worksheet With Answers Chemistry Gas Laws Lab Answers In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Answer questions in complete sentences and support with data from lab. As the bubbles rise, the pressure decreases, so their volume increases. Chemistry Gas Laws Lab Answers.
From www.semanarioangolense.net
Combined Gas Law Chem Worksheet 143 Answer Key » Semanario Worksheet for Student Chemistry Gas Laws Lab Answers Complete all sections of this report and answer all questions in complete sentences for full credit. Applying these laws to compare gases under two different sets of conditions gives the formulas: In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). As the bubbles rise, the pressure decreases, so their volume. Chemistry Gas Laws Lab Answers.
From www.slideshare.net
Gas law packet answers Chemistry Gas Laws Lab Answers A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. Applying these laws to compare gases under two different sets of conditions gives the formulas: Experiment 11 the gas laws. Which gas law is this experiment investigating? In this simulation,. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved help! please answer 2 and graph the data. ( v stands Chemistry Gas Laws Lab Answers Which gas law is this experiment investigating? Applying these laws to compare gases under two different sets of conditions gives the formulas: Complete all sections of this report and answer all questions in complete sentences for full credit. As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. Answer questions in complete sentences and. Chemistry Gas Laws Lab Answers.
From www.pearson.com
5 Ideal Gas Law Experiments PV=nRT or PV=NkT Pearson+ Channels Chemistry Gas Laws Lab Answers Answer questions in complete sentences and support with data from lab. Experiment 11 the gas laws. Which gas law is this experiment investigating? Complete all sections of this report and answer all questions in complete sentences for full credit. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. As the. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Lab+6 gas laws lab Principles of Chemistry Lab 5 Properties of Gases Purpose To study the Chemistry Gas Laws Lab Answers As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. Experiment 11 the gas laws. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment you will (1) determine whether boyle’s. Chemistry Gas Laws Lab Answers.
From studylib.net
Gas Laws Worksheet Answer Key Chemistry Gas Laws Lab Answers In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. As the bubbles rise, the pressure decreases, so their volume increases as suggested by boyle’s law. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Applying these laws to compare gases. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Lab report 6.05 lab Gas Laws Lab Instructions Complete all sections of this report and Chemistry Gas Laws Lab Answers Answer questions in complete sentences and support with data from lab. Complete all sections of this report and answer all questions in complete sentences for full credit. Which gas law is this experiment investigating? Applying these laws to compare gases under two different sets of conditions gives the formulas: In this simulation, students will investigate three of the fundamental gas. Chemistry Gas Laws Lab Answers.
From learninglistlang.z19.web.core.windows.net
Chemistry Gas Laws Worksheet Answers Chemistry Gas Laws Lab Answers P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. Applying these laws to compare gases under two different sets of. Chemistry Gas Laws Lab Answers.
From www.studocu.com
Gas+laws+Lab+2 Lab Experiment 2 Experiment 2 (1 week) (GAS) Gas Laws Purpose The purpose of Chemistry Gas Laws Lab Answers Applying these laws to compare gases under two different sets of conditions gives the formulas: In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and gay. A gas will act like an ideal. Chemistry Gas Laws Lab Answers.
From www.chegg.com
Solved LAB LAB REPORT SHEET Gas Laws 12 A. Boyle's Law Px... Chemistry Gas Laws Lab Answers Applying these laws to compare gases under two different sets of conditions gives the formulas: Answer questions in complete sentences and support with data from lab. Complete all sections of this report and answer all questions in complete sentences for full credit. P1v1=p2v2 , v1/t1=v2/t2 , p1/t1 =p2/t2. In this simulation, students will investigate three of the fundamental gas laws,. Chemistry Gas Laws Lab Answers.
From studylib.net
chem preap Gas laws practice test with answers Chemistry Gas Laws Lab Answers Answer questions in complete sentences and support with data from lab. Applying these laws to compare gases under two different sets of conditions gives the formulas: Complete all sections of this report and answer all questions in complete sentences for full credit. In this simulation, students will investigate three of the fundamental gas laws, including boyle’s law, charles’ law and. Chemistry Gas Laws Lab Answers.
From marlongokegarza.blogspot.com
Phet Gas Laws Simulation Lab Worksheet Answers Chemistry Gas Laws Lab Answers Complete all sections of this report and answer all questions in complete sentences for full credit. In this experiment you will (1) determine whether boyle’s law applies to a mixture of gases (air) and (2). Answer questions in complete sentences and support with data from lab. A gas will act like an ideal gas if its gas molecules are small,. Chemistry Gas Laws Lab Answers.