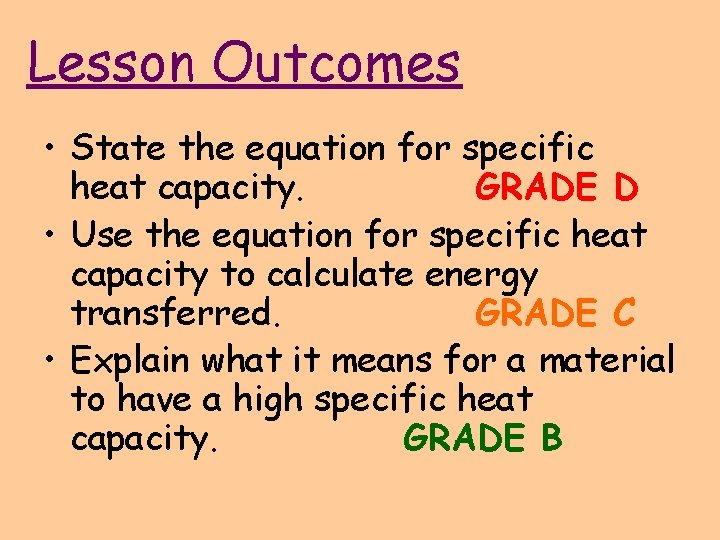
Heat Capacity Of No . The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius.
from slidetodoc.com
The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance.
Specific Heat Capacity The specific heat capacity of
Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas.
From www.slideshare.net
Heat Capacity Heat Capacity Of No Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The heat. Heat Capacity Of No.
From www.slideserve.com
PPT Heat Capacity PowerPoint Presentation, free download ID1128997 Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The heat capacity (c) of a body of matter is. Heat Capacity Of No.
From studymind.co.uk
Heat Capacity Study Mind Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The specific heat (= specific heat. Heat Capacity Of No.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free Heat Capacity Of No The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The specific heat capacity of a substance is. Heat Capacity Of No.
From askfilo.com
The differences between specific heat capacity and heat capacity are.. Heat Capacity Of No Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat (= specific heat. Heat Capacity Of No.
From slidetodoc.com
Specific Heat Capacity The specific heat capacity of Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The specific heat capacity of a. Heat Capacity Of No.
From getrevising.co.uk
Physicsend of year 9 Revision Cards in GCSE Physics Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The heat capacity (c) of a body of matter is. Heat Capacity Of No.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Heat Capacity Of No The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. 55 rows the table of specific heat capacities gives the volumetric heat capacity. Heat Capacity Of No.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Capacity Of No The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity of a substance describes how its temperature changes as. Heat Capacity Of No.
From studylib.net
1.3 Specific Heat Capacity Heat Capacity Of No The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The heat capacity (c) of a body of matter is the quantity of. Heat Capacity Of No.
From www.youtube.com
Heat Capacity and Specific Heat Chemistry Tutorial YouTube Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat (= specific heat capacity) at constant pressure. Heat Capacity Of No.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube Heat Capacity Of No The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat capacity of a. Heat Capacity Of No.
From www.studocu.com
Perryheatcapacities TABLE 2196 Heat Capacities of and Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The heat capacity of a substance describes how its temperature. Heat Capacity Of No.
From slideplayer.com
Heat capacity and Calorimetry ppt download Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat capacity of a material is the energy. Heat Capacity Of No.
From dxoqtwvpm.blob.core.windows.net
What Is Heat Capacity In Materials at James Eng blog Heat Capacity Of No Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The specific. Heat Capacity Of No.
From slideplayer.com
L 19 Thermodynamics [4] Heat capacity ppt download Heat Capacity Of No The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity of a substance describes how its. Heat Capacity Of No.
From www.slideserve.com
PPT Chapter 17 The first law of thermodynamics PowerPoint Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The heat capacity (c) of a body of matter is the. Heat Capacity Of No.
From www.slideserve.com
PPT Heat capacity and specific heat capacity PowerPoint Presentation Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity of a substance describes how. Heat Capacity Of No.
From www.studypool.com
SOLUTION Specific heat capacity definition, importance, and formula Heat Capacity Of No The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat capacity of a material is the energy required. Heat Capacity Of No.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Of No Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). 55 rows the table of specific heat capacities. Heat Capacity Of No.
From www.slideserve.com
PPT Thermal Properties of Matter PowerPoint Presentation, free Heat Capacity Of No The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The heat capacity (c) of a body of matter is the quantity of. Heat Capacity Of No.
From mytutorsource.hk
Learn About Specific Heat Capacity in Less Than 10 Minutes! Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The heat capacity (c) of a body of matter is. Heat Capacity Of No.
From serc.carleton.edu
Heat Capacity Heat Capacity Of No The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. The heat capacity (c) of a body of matter is the quantity of. Heat Capacity Of No.
From www.studypool.com
SOLUTION Physics notes heat capacity and specific heat capacity Heat Capacity Of No Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. The specific heat capacity of a material is. Heat Capacity Of No.
From www.slideshare.net
Heat Capacity Heat Capacity Of No The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change. The heat capacity of a substance describes how. Heat Capacity Of No.
From slidetodoc.com
Specific Heat Capacity The specific heat capacity of Heat Capacity Of No The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. Heat capacity or thermal capacity is. Heat Capacity Of No.
From gcsephysicsninja.com
40. Thermal capacity vs specific heat capacity Heat Capacity Of No The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat (= specific heat capacity) at constant pressure. Heat Capacity Of No.
From www.linstitute.net
AQA A Level Physics复习笔记6.4.4 Latent Heat Capacity翰林国际教育 Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The specific heat (= specific heat capacity) at constant pressure. Heat Capacity Of No.
From www.tes.com
Specific Heat Capacity Poster (Change in Thermal Energy) Teaching Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. The heat capacity (c) of a body of matter is. Heat Capacity Of No.
From www.studocu.com
Lab 2 Specific Heat Capacity 2 Physics 4C Spring 2023 Lab 2 Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The heat capacity of a substance describes how its temperature. Heat Capacity Of No.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a material is the energy required to raise one kilogram (kg) of the material by one degree celsius (°c). The specific heat capacity of a substance is the amount of thermal. Heat Capacity Of No.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The heat capacity (c) of a body of matter is the quantity of heat (q) it absorbs or releases when it experiences a temperature change (δt) of 1 degree celsius. The specific heat (= specific heat. Heat Capacity Of No.
From www.careerpower.in
What is Heat Capacity Definition, Equations, Examples and Types Heat Capacity Of No The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and individual gas. The heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it is the capacity of a substance. The specific heat capacity of a substance is the amount of thermal energy. Heat Capacity Of No.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Heat Capacity Of No The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity or thermal capacity is a physical property of. Heat Capacity Of No.
From edumentors.co.uk
GCSE Physics Particle model of matter Edumentors Heat Capacity Of No 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat capacity of a substance is the amount of thermal energy needed to increase the temperature of 1 kg of the substance by 1°c. The heat capacity (c) of a body of matter is. Heat Capacity Of No.