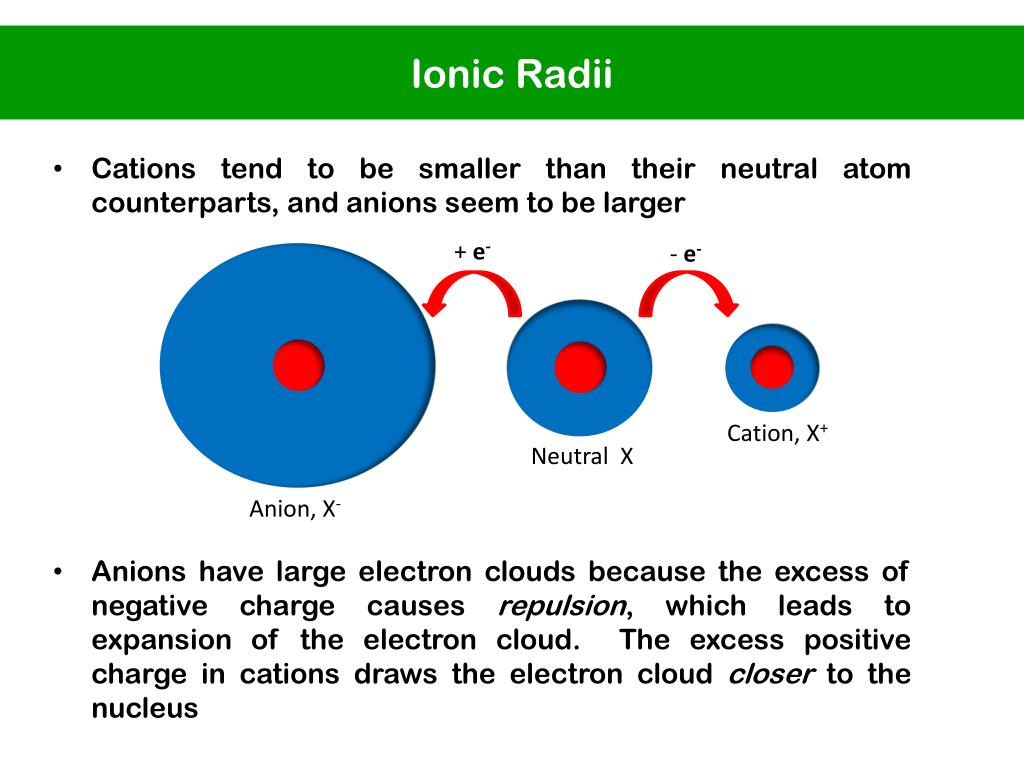
What Is The Cations Of Ionic Radii . — what is ionic radius? — the ionic radius (plural: the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. The radius of a cation is always smaller than the radius of the. cations are formed when an atom loses an electron (s) which results in decreasing the radius. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. To find the value, ions are treated as if they were hard spheres. cations will find arrangements in which they can contact the largest number of anions. Ionic radii) is the measure of an atom's ion in a crystal lattice. Although neither atoms nor ions have. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. If the cation can touch all of its nearest. Ions are formed when an atom loses or gains electrons. — the ionic radius is half the distance between atomic ions in a crystal lattice.
from www.slideserve.com
The radius of a cation is always smaller than the radius of the. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. Ionic radii) is the measure of an atom's ion in a crystal lattice. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. Although neither atoms nor ions have. cations will find arrangements in which they can contact the largest number of anions. — the ionic radius is half the distance between atomic ions in a crystal lattice. To find the value, ions are treated as if they were hard spheres. — the ionic radius (plural: 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure.
PPT LECTURE 7 IONIC COMPOUNDS (Ch. 6) PowerPoint Presentation, free
What Is The Cations Of Ionic Radii The radius of a cation is always smaller than the radius of the. — what is ionic radius? The radius of a cation is always smaller than the radius of the. To find the value, ions are treated as if they were hard spheres. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. — the ionic radius is half the distance between atomic ions in a crystal lattice. — the ionic radius (plural: cations are formed when an atom loses an electron (s) which results in decreasing the radius. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. Ionic radii) is the measure of an atom's ion in a crystal lattice. cations will find arrangements in which they can contact the largest number of anions. If the cation can touch all of its nearest. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. Although neither atoms nor ions have. 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. It is half the distance between two ions that are barely.
From www.numerade.com
SOLVED For ionic materials, assume minimum radius ratio 0.414 for What Is The Cations Of Ionic Radii cations are formed when an atom loses an electron (s) which results in decreasing the radius. The radius of a cation is always smaller than the radius of the. If the cation can touch all of its nearest. Although neither atoms nor ions have. When an atom loses an electron it forms a cation and when it gains an. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT CHAPTER 6 PowerPoint Presentation, free download ID7004913 What Is The Cations Of Ionic Radii Although neither atoms nor ions have. cations are formed when an atom loses an electron (s) which results in decreasing the radius. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. — what is ionic radius? cations will find arrangements in which they can contact the. What Is The Cations Of Ionic Radii.
From chemistryguru.com.sg
How to Explain Atomic and Ionic Radii of Period 3 Elements What Is The Cations Of Ionic Radii It is half the distance between two ions that are barely. 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. cations are formed when an atom loses an electron (s) which results in decreasing the radius. Ions are formed when an atom loses or gains electrons. If the cation. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID2834450 What Is The Cations Of Ionic Radii 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. If the cation can touch all of its nearest. cations will find arrangements in which they can contact the largest number of anions. — the ionic radius is half the distance between atomic ions in a crystal lattice. The. What Is The Cations Of Ionic Radii.
From www.chegg.com
Solved Table 12.3 lonic Radii for Several Cations and Anions What Is The Cations Of Ionic Radii To find the value, ions are treated as if they were hard spheres. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. Although neither atoms nor ions have. If the cation can touch all of its nearest. The radius of a cation is always smaller than the. What Is The Cations Of Ionic Radii.
From secrets-of-periodic-table.blogspot.com
PERIODIC TABLE ELECTRONEGATIVITY NOBLE GASES IONIC RADIUS What Is The Cations Of Ionic Radii Ions are formed when an atom loses or gains electrons. cations will find arrangements in which they can contact the largest number of anions. 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. cations are formed when an atom loses an electron (s) which results in decreasing the. What Is The Cations Of Ionic Radii.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences What Is The Cations Of Ionic Radii When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. cations will find arrangements in which they can contact the largest number of anions. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. The radius of. What Is The Cations Of Ionic Radii.
From www.ck12.org
Ionic Radii CK12 Foundation What Is The Cations Of Ionic Radii cations will find arrangements in which they can contact the largest number of anions. It is half the distance between two ions that are barely. — what is ionic radius? Although neither atoms nor ions have. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. Ions are. What Is The Cations Of Ionic Radii.
From sciencenotes.org
Atomic Radius and Ionic Radius What Is The Cations Of Ionic Radii Although neither atoms nor ions have. 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. cations will find arrangements in which they can contact the largest number of anions. — the ionic radius is half the distance between atomic ions in a crystal lattice. Ionic radius is the. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Periodic properties of the elements PowerPoint Presentation, free What Is The Cations Of Ionic Radii If the cation can touch all of its nearest. To find the value, ions are treated as if they were hard spheres. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. It is half the distance between two ions that are barely. cations will find arrangements. What Is The Cations Of Ionic Radii.
From inspiritvr.com
Ionic radii Study Guide Inspirit Learning Inc What Is The Cations Of Ionic Radii 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. — the ionic radius is half the distance between atomic ions in a crystal lattice. To find the value, ions are treated as if they were hard spheres. — what is ionic radius? It is half the distance between. What Is The Cations Of Ionic Radii.
From printablepovedenu4k.z4.web.core.windows.net
Atomic Radius Trend Explanation What Is The Cations Of Ionic Radii Although neither atoms nor ions have. To find the value, ions are treated as if they were hard spheres. Ions are formed when an atom loses or gains electrons. The radius of a cation is always smaller than the radius of the. It is half the distance between two ions that are barely. Ionic radii) is the measure of an. What Is The Cations Of Ionic Radii.
From headinghometodinner.org
Radio iónico Introducción a la química Heading What Is The Cations Of Ionic Radii It is half the distance between two ions that are barely. — the ionic radius (plural: 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. To find the value, ions are treated as if they were hard spheres. Although neither atoms nor ions have. Ionic radii) is the measure. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Atomic Orbitals PowerPoint Presentation, free download ID1804014 What Is The Cations Of Ionic Radii 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. — what is ionic radius? Ions are formed when an atom loses or gains electrons. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. The radius of. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Chemical Periodicity PowerPoint Presentation, free download ID What Is The Cations Of Ionic Radii — what is ionic radius? When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. To find the value, ions are treated as if they were hard spheres. — the ionic radius (plural: cations will find arrangements in which they can contact the largest number of anions.. What Is The Cations Of Ionic Radii.
From slidetodoc.com
Radius ratio In ionic solids cations try to What Is The Cations Of Ionic Radii cations will find arrangements in which they can contact the largest number of anions. Ionic radii) is the measure of an atom's ion in a crystal lattice. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. 100 rows — ionic radius, rion, is the radius. What Is The Cations Of Ionic Radii.
From www.researchgate.net
Simplified representation of the ionic radii and the net electric What Is The Cations Of Ionic Radii the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. cations will find arrangements in which they can contact the largest number of anions. The. What Is The Cations Of Ionic Radii.
From sscchemistry.weebly.com
Periodic Trends SSC Chemistry What Is The Cations Of Ionic Radii Ionic radii) is the measure of an atom's ion in a crystal lattice. The radius of a cation is always smaller than the radius of the. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. cations are formed when an atom loses an electron (s) which results in. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Anatomy of the Periodic Table PowerPoint Presentation, free What Is The Cations Of Ionic Radii It is half the distance between two ions that are barely. — the ionic radius (plural: To find the value, ions are treated as if they were hard spheres. cations are formed when an atom loses an electron (s) which results in decreasing the radius. 100 rows — ionic radius, rion, is the radius of a monatomic. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 What Is The Cations Of Ionic Radii the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. The radius of a cation is always smaller than the radius of the. To find the value, ions are treated as if they were hard spheres. — the ionic radius is half the distance between atomic ions. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT LECTURE 7 IONIC COMPOUNDS (Ch. 6) PowerPoint Presentation, free What Is The Cations Of Ionic Radii If the cation can touch all of its nearest. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. — what is ionic radius? It is half. What Is The Cations Of Ionic Radii.
From www.researchgate.net
Ionic radii of cations and anions in olivine as a function of oxidation What Is The Cations Of Ionic Radii Ions are formed when an atom loses or gains electrons. — the ionic radius is half the distance between atomic ions in a crystal lattice. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. — the ionic radius (plural: 100 rows — ionic radius,. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 What Is The Cations Of Ionic Radii It is half the distance between two ions that are barely. Ionic radii) is the measure of an atom's ion in a crystal lattice. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. 100 rows — ionic radius, rion, is the radius of a monatomic ion. What Is The Cations Of Ionic Radii.
From www.chegg.com
Solved Table 3.4 Ionic Radii for Several Cations and Anions What Is The Cations Of Ionic Radii When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. cations are formed when an atom loses an electron (s) which results in decreasing the radius. The radius of a cation is always smaller than the radius of the. Ionic radii) is the measure of an atom's ion in. What Is The Cations Of Ionic Radii.
From elchoroukhost.net
Periodic Table Ionic Radius Elcho Table What Is The Cations Of Ionic Radii — the ionic radius (plural: It is half the distance between two ions that are barely. The radius of a cation is always smaller than the radius of the. 100 rows — ionic radius, rion, is the radius of a monatomic ion in an ionic crystal structure. cations will find arrangements in which they can contact the. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT LECTURE 7 IONIC COMPOUNDS (Ch. 6) PowerPoint Presentation, free What Is The Cations Of Ionic Radii — the ionic radius (plural: The radius of a cation is always smaller than the radius of the. Although neither atoms nor ions have. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. Ionic radius is the distance from the nucleus of an ion up to which it. What Is The Cations Of Ionic Radii.
From ar.inspiredpencil.com
Ionic Radius Diagram What Is The Cations Of Ionic Radii cations will find arrangements in which they can contact the largest number of anions. To find the value, ions are treated as if they were hard spheres. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. the ionic radii of cations and anions are always smaller or. What Is The Cations Of Ionic Radii.
From inspiritvr.com
Ionic radii Study Guide Inspirit Learning Inc What Is The Cations Of Ionic Radii — the ionic radius is half the distance between atomic ions in a crystal lattice. — the ionic radius (plural: It is half the distance between two ions that are barely. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. — what is ionic. What Is The Cations Of Ionic Radii.
From anthonystrendsassignment.weebly.com
Ionic Radius Periodic Trends What Is The Cations Of Ionic Radii Ions are formed when an atom loses or gains electrons. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. 100 rows — ionic radius,. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Chemical Periodicity PowerPoint Presentation, free download ID What Is The Cations Of Ionic Radii — the ionic radius is half the distance between atomic ions in a crystal lattice. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud.. What Is The Cations Of Ionic Radii.
From chem.libretexts.org
8.2 Atomic and Ionic Radius Chemistry LibreTexts What Is The Cations Of Ionic Radii The radius of a cation is always smaller than the radius of the. Ions are formed when an atom loses or gains electrons. To find the value, ions are treated as if they were hard spheres. When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. It is half the. What Is The Cations Of Ionic Radii.
From printablemimsie715j6.z22.web.core.windows.net
Cations And Ions Explained Chart What Is The Cations Of Ionic Radii — what is ionic radius? Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. cations will find arrangements in which they can contact the largest number of anions. When an atom loses an electron it forms a cation and when it gains an electron it. What Is The Cations Of Ionic Radii.
From answerlisthydra.z21.web.core.windows.net
How Do Ions Form Ionic Bonds What Is The Cations Of Ionic Radii It is half the distance between two ions that are barely. the ionic radii of cations and anions are always smaller or larger, respectively, than the parent atom due to changes in. cations are formed when an atom loses an electron (s) which results in decreasing the radius. 100 rows — ionic radius, rion, is the radius. What Is The Cations Of Ionic Radii.
From www.pathwaystochemistry.com
Atomic and Ionic Radii Pathways to Chemistry What Is The Cations Of Ionic Radii The radius of a cation is always smaller than the radius of the. If the cation can touch all of its nearest. It is half the distance between two ions that are barely. Ionic radii) is the measure of an atom's ion in a crystal lattice. 100 rows — ionic radius, rion, is the radius of a monatomic ion. What Is The Cations Of Ionic Radii.
From www.slideserve.com
PPT Introduction to the Periodic Table PowerPoint Presentation, free What Is The Cations Of Ionic Radii When an atom loses an electron it forms a cation and when it gains an electron it becomes an anion. It is half the distance between two ions that are barely. Ionic radius is the distance from the nucleus of an ion up to which it has an influence on its electron cloud. Ions are formed when an atom loses. What Is The Cations Of Ionic Radii.