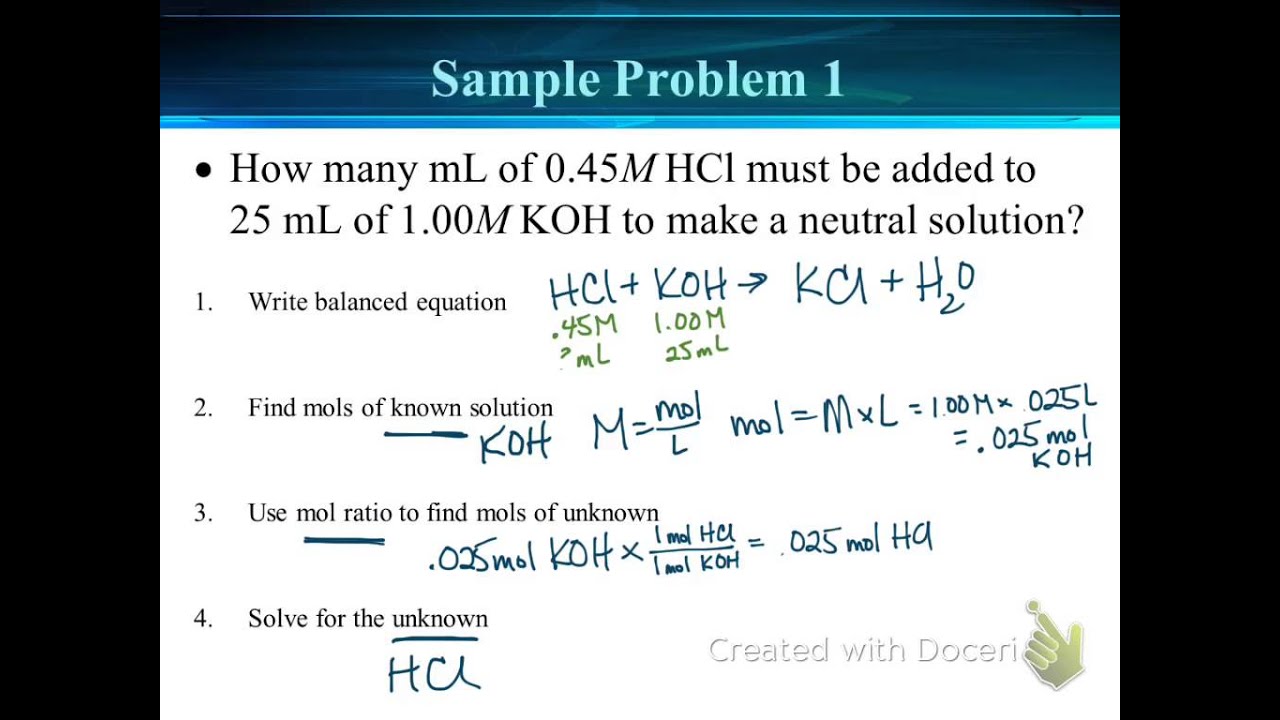
Titration Reaction Calculations . The following examples work through these kinds of titration calculations: Titration calculations are used to find the concentration of unknown solutions; First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. Calculating ph for titration solutions: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. They can also be used to calculate the ph after a given point during a titration The volumes of acids and alkali solutions that react with each other can be measured by.
from www.youtube.com
Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). The following examples work through these kinds of titration calculations: To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. The amount of added titrant is determined from its concentration and volume: They can also be used to calculate the ph after a given point during a titration The volumes of acids and alkali solutions that react with each other can be measured by. First determine the moles of \(\ce{naoh}\) in the reaction. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculating ph for titration solutions:
Solving AcidBase Titration Problems YouTube
Titration Reaction Calculations To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. First determine the moles of \(\ce{naoh}\) in the reaction. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The following examples work through these kinds of titration calculations: They can also be used to calculate the ph after a given point during a titration Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). Calculating ph for titration solutions: Titration calculations are used to find the concentration of unknown solutions; N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The amount of added titrant is determined from its concentration and volume: To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. The volumes of acids and alkali solutions that react with each other can be measured by.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Reaction Calculations From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). First determine the moles of \(\ce{naoh}\) in the reaction. Titration is a method to determine the. Titration Reaction Calculations.
From www.youtube.com
Water Hardness (EDTA) Titration Calculations Example YouTube Titration Reaction Calculations The volumes of acids and alkali solutions that react with each other can be measured by. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. They can also be used to calculate the ph after a given point during a titration N (mol) = c (mol /l) * v (l) and the amount of. Titration Reaction Calculations.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Reaction Calculations Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Titration calculations are used to find the concentration of unknown solutions; N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Strong acid/strong base a titration is carried out for 25.00 ml of 0.100. Titration Reaction Calculations.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Reaction Calculations The volumes of acids and alkali solutions that react with each other can be measured by. The following examples work through these kinds of titration calculations: Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). First. Titration Reaction Calculations.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Reaction Calculations Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Calculating ph for titration solutions: They can also be used to calculate the ph after a given point during a titration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The volumes of. Titration Reaction Calculations.
From www.numerade.com
SOLVED The balanced equation for the titration of oxalic acid with Titration Reaction Calculations The volumes of acids and alkali solutions that react with each other can be measured by. The following examples work through these kinds of titration calculations: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration calculations. Titration Reaction Calculations.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Reaction Calculations First determine the moles of \(\ce{naoh}\) in the reaction. They can also be used to calculate the ph after a given point during a titration The amount of added titrant is determined from its concentration and volume: Calculating ph for titration solutions: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Strong acid/strong base. Titration Reaction Calculations.
From www.studocu.com
Titration Calculations for Acid Base Neutralization Reactions Copy Titration Reaction Calculations Titration calculations are used to find the concentration of unknown solutions; The following examples work through these kinds of titration calculations: To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculating ph for titration. Titration Reaction Calculations.
From www.showme.com
Titration Curves Calculations Science, Chemicalreactions, Chemistry Titration Reaction Calculations They can also be used to calculate the ph after a given point during a titration The following examples work through these kinds of titration calculations: Calculating ph for titration solutions: The volumes of acids and alkali solutions that react with each other can be measured by. Titration calculations are used to find the concentration of unknown solutions; N (mol). Titration Reaction Calculations.
From www.science-revision.co.uk
Titrations Titration Reaction Calculations The following examples work through these kinds of titration calculations: To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The amount of added titrant is determined from its concentration and. Titration Reaction Calculations.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Reaction Calculations Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. They. Titration Reaction Calculations.
From www.youtube.com
A Simple Guide to Titration Calculations Part 1 Concentration YouTube Titration Reaction Calculations The following examples work through these kinds of titration calculations: First determine the moles of \(\ce{naoh}\) in the reaction. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The volumes of acids and alkali solutions that react with each other can be measured by. Titration calculations are used to find the concentration of unknown. Titration Reaction Calculations.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Reaction Calculations To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of. Titration Reaction Calculations.
From www.edplace.com
Understand Advanced Titration Calculations Worksheet EdPlace Titration Reaction Calculations Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. First determine the moles of \(\ce{naoh}\) in the reaction. To calculate ph at any point in a titration, the amounts of all species must. Titration Reaction Calculations.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Reaction Calculations The amount of added titrant is determined from its concentration and volume: Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). Calculating ph for titration solutions: Titration calculations are used to find the concentration of unknown. Titration Reaction Calculations.
From www.youtube.com
Calculations for Titration of Weak Base with Strong Acid, initial pH Titration Reaction Calculations The volumes of acids and alkali solutions that react with each other can be measured by. The following examples work through these kinds of titration calculations: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. To calculate ph at any point in a titration, the amounts of all species must first be determined using. Titration Reaction Calculations.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Reaction Calculations To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration calculations are used to find the concentration of unknown solutions; The amount of added. Titration Reaction Calculations.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Reaction Calculations From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Titration calculations are used to find the concentration of unknown solutions; The amount. Titration Reaction Calculations.
From www.youtube.com
Calculating pH During Titration Reactions YouTube Titration Reaction Calculations To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration calculations are used to find the concentration of unknown. Titration Reaction Calculations.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Reaction Calculations The amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. The volumes of acids and alkali solutions that react with each other can be measured by. Titration calculations are used to find the concentration of unknown solutions;. Titration Reaction Calculations.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2976284 Titration Reaction Calculations First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: Calculating ph for titration solutions: Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). To. Titration Reaction Calculations.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Reaction Calculations The following examples work through these kinds of titration calculations: They can also be used to calculate the ph after a given point during a titration N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. First determine the moles of \(\ce{naoh}\) in the reaction. Titration calculations are used. Titration Reaction Calculations.
From www.showme.com
Example 1 titration calculations Science, Chemistry, Chemical Titration Reaction Calculations The following examples work through these kinds of titration calculations: The amount of added titrant is determined from its concentration and volume: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Calculating ph. Titration Reaction Calculations.
From mungfali.com
Acid Base Titration Calculation Titration Reaction Calculations The amount of added titrant is determined from its concentration and volume: From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The volumes of acids and alkali solutions that react with each other can be measured by. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric.. Titration Reaction Calculations.
From www.nagwa.com
Question Video Calculating the Mass of Solute in a Solution via Titration Reaction Calculations From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. Titration calculations are used to find the concentration of unknown solutions; N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Calculating ph. Titration Reaction Calculations.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Reaction Calculations From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. The following examples work through these kinds of titration calculations: Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). They can also be used to. Titration Reaction Calculations.
From chemistry.stackexchange.com
equilibrium How to calculate the dissociation constant of a weak acid Titration Reaction Calculations The amount of added titrant is determined from its concentration and volume: They can also be used to calculate the ph after a given point during a titration First determine the moles of \(\ce{naoh}\) in the reaction. The following examples work through these kinds of titration calculations: Titration is a method to determine the unknown concentration of a specific substance. Titration Reaction Calculations.
From www.youtube.com
Basic Titrations Chemistry Tutorial YouTube Titration Reaction Calculations N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. Titration calculations are used to find the concentration of unknown solutions; Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve. Titration Reaction Calculations.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Reaction Calculations Strong acid/strong base a titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link] ). First determine the moles of \(\ce{naoh}\) in the reaction. The volumes of acids and alkali solutions that react with each other can be measured by. The amount. Titration Reaction Calculations.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Reaction Calculations To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. The volumes of acids and alkali solutions that react with each other can be measured by. First determine the moles of \(\ce{naoh}\) in the reaction. The following examples work through these kinds of titration calculations: The. Titration Reaction Calculations.
From mavink.com
Acid Base Titration Equation Titration Reaction Calculations Calculating ph for titration solutions: First determine the moles of \(\ce{naoh}\) in the reaction. The amount of added titrant is determined from its concentration and volume: The volumes of acids and alkali solutions that react with each other can be measured by. The following examples work through these kinds of titration calculations: From the mole ratio, calculate the moles of. Titration Reaction Calculations.
From dxopmiczw.blob.core.windows.net
How To Do Titration Calculations A Level Chemistry at Lorraine Nix blog Titration Reaction Calculations The amount of added titrant is determined from its concentration and volume: Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual stoichiometric. They can also be used to calculate the ph after a given. Titration Reaction Calculations.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube Titration Reaction Calculations The following examples work through these kinds of titration calculations: First determine the moles of \(\ce{naoh}\) in the reaction. Titration is a method to determine the unknown concentration of a specific substance (analyte) dissolved. The volumes of acids and alkali solutions that react with each other can be measured by. N (mol) = c (mol /l) * v (l) and. Titration Reaction Calculations.
From www.youtube.com
Learn how to calculate the average mass of aspirin in tablets using a Titration Reaction Calculations From the mole ratio, calculate the moles of \(\ce{h_2so_4}\) that reacted. Calculating ph for titration solutions: The following examples work through these kinds of titration calculations: The volumes of acids and alkali solutions that react with each other can be measured by. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in. Titration Reaction Calculations.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Reaction Calculations They can also be used to calculate the ph after a given point during a titration To calculate ph at any point in a titration, the amounts of all species must first be determined using the stoichiometry of the neutralization. First determine the moles of \(\ce{naoh}\) in the reaction. The following examples work through these kinds of titration calculations: N. Titration Reaction Calculations.