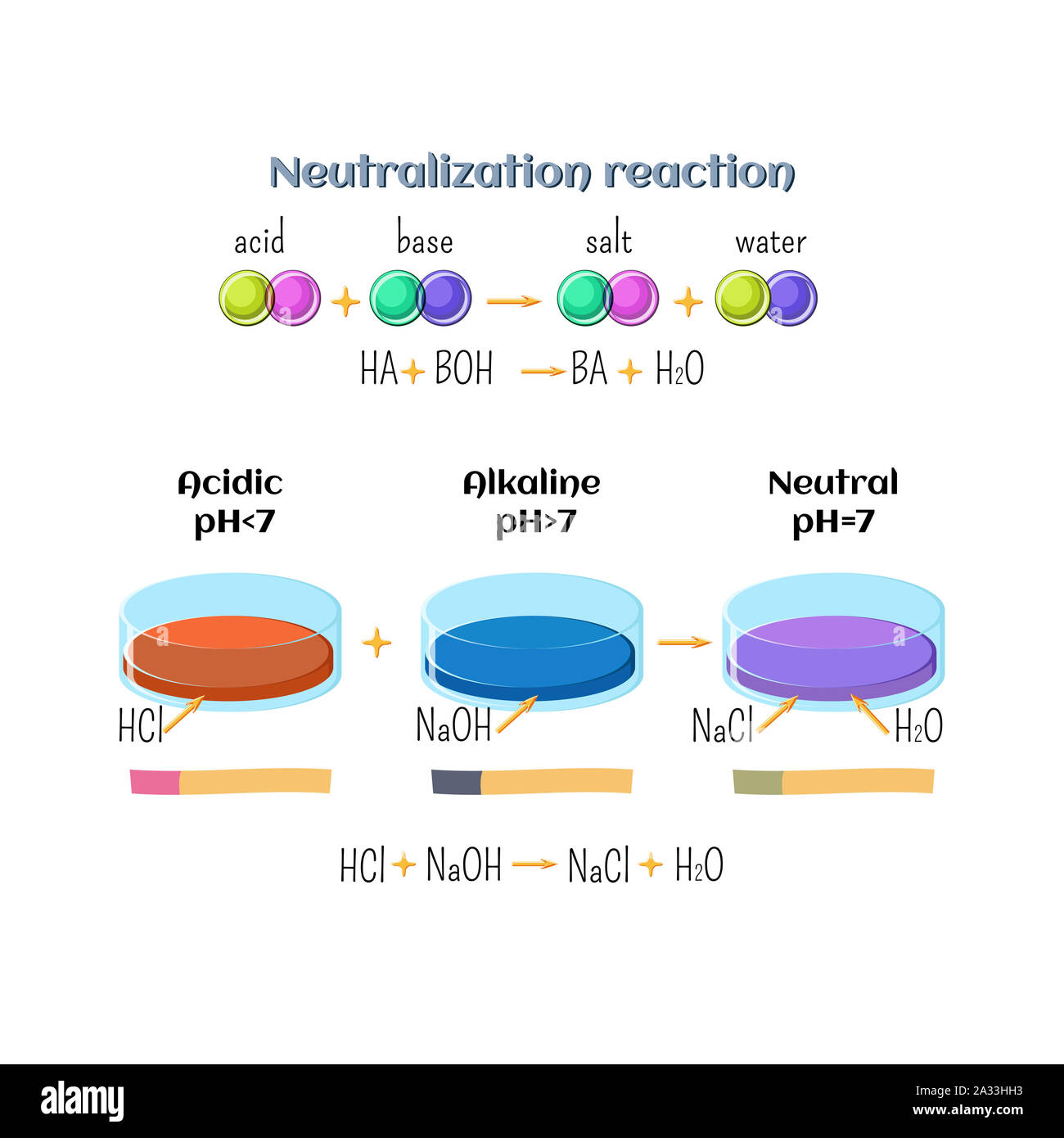
Neutralization Indicators Examples . Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. For example, red cabbage juice contains a mixture of colored. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Indicators are used to determine whether a solution is acidic or alkaline. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine if a solution is acidic or alkaline. Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Substances such as phenolphthalein, which can be.
from www.alamy.com
The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. For example, red cabbage juice contains a mixture of colored. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Acids react with metals, bases and carbonates to produce salts.
Acidbase neutralization reaction, illustration Stock Photo Alamy
Neutralization Indicators Examples Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. Acids react with metals, bases and carbonates to produce salts. For example, red cabbage juice contains a mixture of colored. Acids react with metals, bases and carbonates to produce salts. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine whether a solution is acidic or alkaline. Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. Many different substances can be used as indicators, depending on the particular reaction to be monitored. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Indicators are used to determine if a solution is acidic or alkaline.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo Neutralization Indicators Examples Neutralisation is the reaction between an acid and a base. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. For example, red cabbage juice contains a mixture of colored. Acids react with. Neutralization Indicators Examples.
From www.slideserve.com
PPT Titration and AcidBase Neutralization PowerPoint Presentation Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Substances such as phenolphthalein, which can be. For example, red cabbage juice contains a mixture of colored. Indicators are used to determine whether a solution is acidic or alkaline. The color of acid base indicators varies in acidic. Neutralization Indicators Examples.
From www.youtube.com
Neutralization Reaction Acids and Bases, Class 7 Physics Digital Neutralization Indicators Examples Indicators are used to determine whether a solution is acidic or alkaline. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. For example, red cabbage juice contains a mixture of colored. Acids react with metals, bases and carbonates to produce salts. The color of. Neutralization Indicators Examples.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Neutralization Indicators Examples Acids react with metals, bases and carbonates to produce salts. For example, red cabbage juice contains a mixture of colored. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Substances such as phenolphthalein, which can be. Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or. Neutralization Indicators Examples.
From stock.adobe.com
Neutralization Reaction Infographic Diagram with example of Neutralization Indicators Examples Many different substances can be used as indicators, depending on the particular reaction to be monitored. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Substances such as phenolphthalein, which can be. Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts.. Neutralization Indicators Examples.
From slideplayer.com
Neutralization, Indicators, and Titrations ppt download Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are used to. Neutralization Indicators Examples.
From www.slideserve.com
PPT 7E Acids and alkalis PowerPoint Presentation, free download ID Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Many different substances can be used as indicators, depending on the. Neutralization Indicators Examples.
From slideplayer.com
Acid and Bases Chapter ppt download Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Substances such as phenolphthalein, which can be. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Acids react with metals, bases and carbonates to produce salts. In more basic solutions where the hydronium ion concentration is less than. Neutralization Indicators Examples.
From studiousguy.com
7 Neutralization Examples in Everyday Life StudiousGuy Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. For example, red cabbage juice contains a mixture of colored. Neutralisation is the reaction between an acid and a base. Substances such as. Neutralization Indicators Examples.
From trendzhit.com
Neutralisation Reaction Defination, Types, Equation, Applications Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Indicators are used to determine if a solution is acidic or alkaline. Indicators are used to determine if a solution is acidic or alkaline. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it. Neutralization Indicators Examples.
From dokumen.tips
(PPTX) acidbase indicator and neutralization DOKUMEN.TIPS Neutralization Indicators Examples In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. For example, red. Neutralization Indicators Examples.
From sciencenotes.org
Neutralization Reaction Definition and Products Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. For example, red cabbage juice contains a mixture of colored. Substances such as. Neutralization Indicators Examples.
From www.youtube.com
Unit 15.4 Indicators, Neutralization Reactions, & Using the Titration Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Substances such as phenolphthalein, which can be. For example, red cabbage juice contains a mixture of colored. Indicators are used to determine if a solution is acidic or alkaline. Neutralisation is the reaction between an acid and a base. Indicators are used to determine whether. Neutralization Indicators Examples.
From www.studocu.com
Chemistry example 14 Principles of Neutralization Titrations 14A Neutralization Indicators Examples Many different substances can be used as indicators, depending on the particular reaction to be monitored. Neutralisation is the reaction between an acid and a base. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine if a solution is. Neutralization Indicators Examples.
From studygoddessliaa0.z21.web.core.windows.net
Give An Example Of A Neutralization Reaction Neutralization Indicators Examples In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. Indicators are used to determine whether a solution is acidic or alkaline. Substances such as. Neutralization Indicators Examples.
From www.teachoo.com
[Class 7] Neutralisation Reaction Concept, Formula Teachoo Neutralization Indicators Examples Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. Many different substances can be used as indicators, depending on the particular reaction to be monitored. The color of acid base. Neutralization Indicators Examples.
From www.slideserve.com
PPT PSC 4012 PowerPoint Presentation, free download ID1995395 Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a base. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. For example, red cabbage juice contains. Neutralization Indicators Examples.
From dewwool.com
25 Examples of neutralization reaction DewWool Neutralization Indicators Examples Many different substances can be used as indicators, depending on the particular reaction to be monitored. Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine whether a solution is acidic or alkaline. For example, red cabbage juice contains a mixture of colored. Indicators are. Neutralization Indicators Examples.
From www.slideserve.com
PPT Chemical Reactions in Aqueous Solutions PowerPoint Presentation Neutralization Indicators Examples Acid base indicators are also called hydrogen ion indicators or neutralization indicators. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Indicators are used to determine if a solution is acidic or alkaline. Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. In more. Neutralization Indicators Examples.
From www.chemicals.co.uk
What is Neutralisation in Chemistry? The Chemistry Blog Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. Acids react with metals, bases and carbonates to produce salts. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Many different substances can be used as indicators, depending on the particular reaction to be monitored.. Neutralization Indicators Examples.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID6673080 Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. Substances such as phenolphthalein, which can be. For example, red cabbage juice contains a mixture of. Neutralization Indicators Examples.
From slideplayer.com
Neutralization. ppt download Neutralization Indicators Examples Many different substances can be used as indicators, depending on the particular reaction to be monitored. Acids react with metals, bases and carbonates to produce salts. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine if a solution is acidic or alkaline. Indicators are used to determine if a solution is acidic or alkaline.. Neutralization Indicators Examples.
From www.youtube.com
Acid Base Neutralization Reactions Chemistry YouTube Neutralization Indicators Examples Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Indicators are used to determine if a solution is acidic or alkaline. Substances such as phenolphthalein, which can be. Acids react with metals, bases and carbonates to produce salts. Neutralisation is the reaction between an acid and a. Neutralization Indicators Examples.
From www.slideserve.com
PPT Neutralization PowerPoint Presentation, free download ID1994999 Neutralization Indicators Examples Acids react with metals, bases and carbonates to produce salts. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Indicators are used to determine if a solution is acidic or alkaline. Many different substances can be used as indicators, depending on the particular reaction to be monitored. The color of acid base indicators varies in acidic and. Neutralization Indicators Examples.
From slideplayer.com
Neutralization, Indicators, and Titrations ppt download Neutralization Indicators Examples Substances such as phenolphthalein, which can be. For example, red cabbage juice contains a mixture of colored. Neutralisation is the reaction between an acid and a base. Many different substances can be used as indicators, depending on the particular reaction to be monitored. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m. Neutralization Indicators Examples.
From slideplayer.com
Neutralization, Indicators, and Titrations ppt download Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Acids react with metals, bases and carbonates to produce salts. For example, red cabbage juice contains a mixture of colored. Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or alkaline. Acid base indicators. Neutralization Indicators Examples.
From www.alamy.com
Acidbase neutralization reaction, illustration Stock Photo Alamy Neutralization Indicators Examples Acids react with metals, bases and carbonates to produce salts. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Neutralisation is the reaction between an acid and a base. For example, red cabbage juice contains a mixture of colored. In more basic solutions where the hydronium ion concentration is less than 5.0 ×. Neutralization Indicators Examples.
From www.slideserve.com
PPT Chapter 6 Solutions, Acids, and Bases PowerPoint Presentation Neutralization Indicators Examples Neutralisation is the reaction between an acid and a base. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Acids react with metals, bases and carbonates to produce salts. For example, red cabbage juice contains a mixture of colored. Substances such as phenolphthalein, which can be. The color of acid base indicators varies. Neutralization Indicators Examples.
From acidsandbases-science.blogspot.com
Acids and Bases Lemon Juice and Toothpaste Neutralization Neutralization Indicators Examples For example, red cabbage juice contains a mixture of colored. Indicators are used to determine if a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Indicators are used to determine if a solution is acidic or alkaline.. Neutralization Indicators Examples.
From www.slideserve.com
PPT PSC 4012 PowerPoint Presentation, free download ID1995395 Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Indicators are used to determine if a solution is acidic or alkaline. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Acids react with metals, bases. Neutralization Indicators Examples.
From www.sliderbase.com
Indicators Neutralization Indicators Examples The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Substances such as phenolphthalein, which can be. Neutralisation is the reaction between an acid and a base. Indicators are used to determine if a solution is acidic or alkaline. For example, red cabbage juice contains a mixture of colored. In more basic solutions where the. Neutralization Indicators Examples.
From acidsandbases-science.blogspot.com
Acids and Bases Lemon Juice and Toothpaste Neutralization Neutralization Indicators Examples Indicators are used to determine if a solution is acidic or alkaline. Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Acids react with metals, bases and carbonates to produce salts. In. Neutralization Indicators Examples.
From slideplayer.com
Neutralization, Indicators, and Titrations ppt download Neutralization Indicators Examples Acid base indicators are also called hydrogen ion indicators or neutralization indicators. Substances such as phenolphthalein, which can be. Indicators are used to determine whether a solution is acidic or alkaline. Acids react with metals, bases and carbonates to produce salts. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph >. Neutralization Indicators Examples.
From slideplayer.com
Neutralization, Indicators, and Titrations ppt download Neutralization Indicators Examples In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acids react with metals, bases and carbonates to produce salts. Many different substances can be used as indicators, depending on the particular reaction to be monitored. Neutralisation is the reaction between an acid and a. Neutralization Indicators Examples.
From slideplayer.com
Neutralization, Indicators, and Titrations ppt download Neutralization Indicators Examples Many different substances can be used as indicators, depending on the particular reaction to be monitored. Substances such as phenolphthalein, which can be. Acid base indicators are also called hydrogen ion indicators or neutralization indicators. The color of acid base indicators varies in acidic and alkaline mediums, that is, their. Acids react with metals, bases and carbonates to produce salts.. Neutralization Indicators Examples.