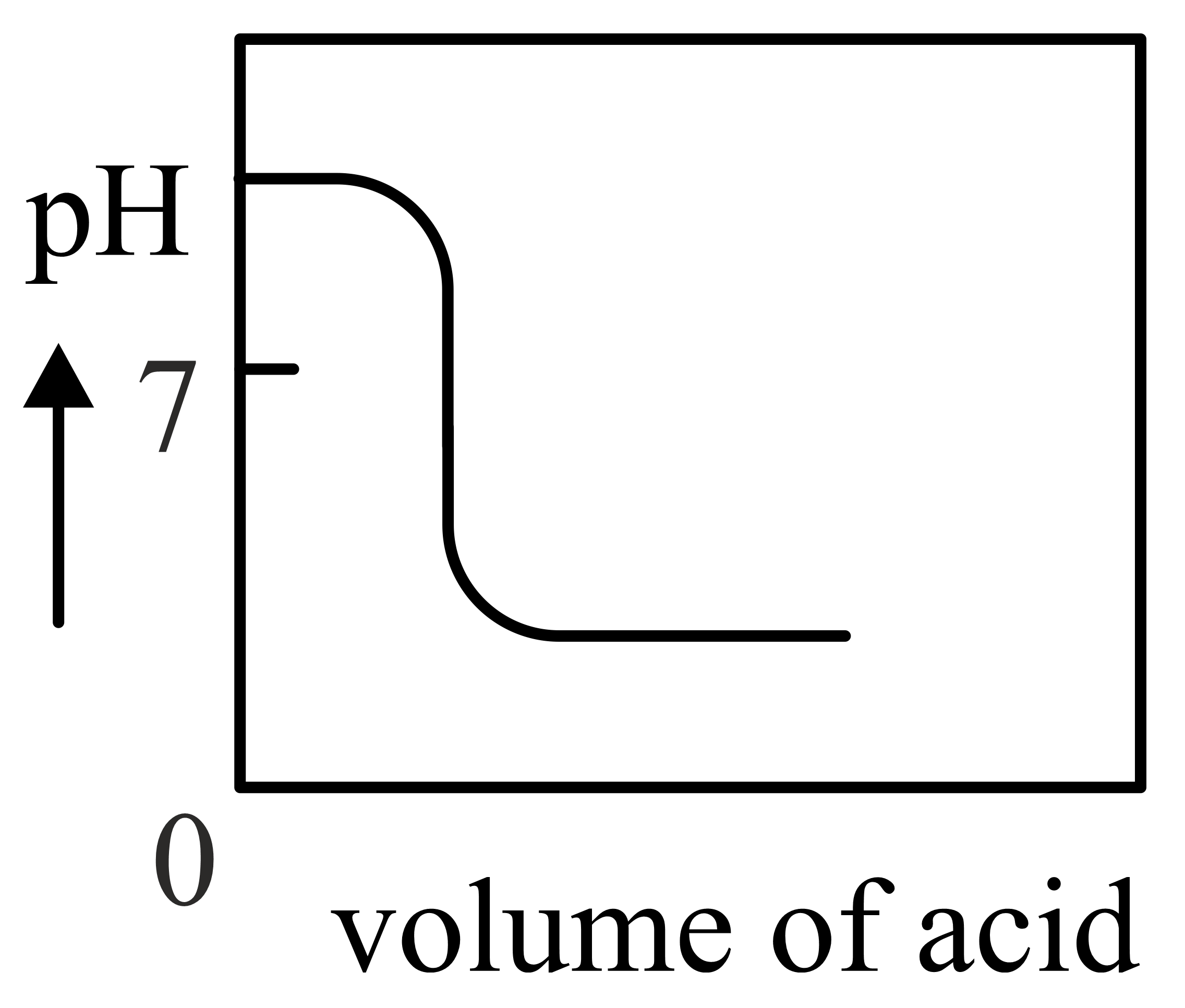
Basic Principles Of Ph Metric Titration . The ph of the solution is plotted versus. when chemical indicators are not suitable, a potentiometric ph titration can also be used. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. conductance or the photometric absorbance, the term linear titration curve is used. a graph of ph (column b) plotted as ordinate vs. Compute sample ph at important stages of a titration; When a logarithmic expression of the. there are two basic types of acid base titrations, indicator and potentiometric. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1.
from www.embibe.com
a graph of ph (column b) plotted as ordinate vs. there are two basic types of acid base titrations, indicator and potentiometric. The ph of the solution is plotted versus. conductance or the photometric absorbance, the term linear titration curve is used. When a logarithmic expression of the. when chemical indicators are not suitable, a potentiometric ph titration can also be used. Compute sample ph at important stages of a titration; the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1.
The Plot of pHmetric titration of weak base NH4OH vs strong acid HCl
Basic Principles Of Ph Metric Titration conductance or the photometric absorbance, the term linear titration curve is used. The ph of the solution is plotted versus. When a logarithmic expression of the. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. Compute sample ph at important stages of a titration; when chemical indicators are not suitable, a potentiometric ph titration can also be used. a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. conductance or the photometric absorbance, the term linear titration curve is used. there are two basic types of acid base titrations, indicator and potentiometric.
From www.youtube.com
pH titration curve calculations for weak acid strong base YouTube Basic Principles Of Ph Metric Titration there are two basic types of acid base titrations, indicator and potentiometric. conductance or the photometric absorbance, the term linear titration curve is used. The ph of the solution is plotted versus. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. a graph of ph (column b) plotted as ordinate vs. When. Basic Principles Of Ph Metric Titration.
From www.researchgate.net
pHMetric Titration Curves of HClO 4 and Different 1Substituted Basic Principles Of Ph Metric Titration there are two basic types of acid base titrations, indicator and potentiometric. when chemical indicators are not suitable, a potentiometric ph titration can also be used. The ph of the solution is plotted versus. When a logarithmic expression of the. Compute sample ph at important stages of a titration; Volume (column a) as abscissa provides a titration curve. Basic Principles Of Ph Metric Titration.
From www.embibe.com
The Plot of pHmetric titration of weak base NH4OH vs strong acid HCl Basic Principles Of Ph Metric Titration conductance or the photometric absorbance, the term linear titration curve is used. when chemical indicators are not suitable, a potentiometric ph titration can also be used. a graph of ph (column b) plotted as ordinate vs. When a logarithmic expression of the. there are two basic types of acid base titrations, indicator and potentiometric. the. Basic Principles Of Ph Metric Titration.
From giosbxlyr.blob.core.windows.net
Ph Titration Curve Equivalence Point at Kimberly Hunter blog Basic Principles Of Ph Metric Titration Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. conductance or the photometric absorbance, the term linear titration curve is used.. Basic Principles Of Ph Metric Titration.
From hxeqfxiks.blob.core.windows.net
How To Use Ph Meter In Titration at Shannon Wright blog Basic Principles Of Ph Metric Titration When a logarithmic expression of the. conductance or the photometric absorbance, the term linear titration curve is used. when chemical indicators are not suitable, a potentiometric ph titration can also be used. a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment are to measure the ph of various solutions using. Basic Principles Of Ph Metric Titration.
From microbenotes.com
pH Meter Principle, Parts, Procedure, Types, Uses, Examples Basic Principles Of Ph Metric Titration When a logarithmic expression of the. there are two basic types of acid base titrations, indicator and potentiometric. when chemical indicators are not suitable, a potentiometric ph titration can also be used. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. a graph of. Basic Principles Of Ph Metric Titration.
From www.youtube.com
Strong Acid Strong Base Titration Curve and pH calculations SA SB Basic Principles Of Ph Metric Titration there are two basic types of acid base titrations, indicator and potentiometric. Compute sample ph at important stages of a titration; a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. Volume (column a) as abscissa. Basic Principles Of Ph Metric Titration.
From www.studypool.com
SOLUTION pH Metric Titration Summary Studypool Basic Principles Of Ph Metric Titration a graph of ph (column b) plotted as ordinate vs. there are two basic types of acid base titrations, indicator and potentiometric. The ph of the solution is plotted versus. Compute sample ph at important stages of a titration; conductance or the photometric absorbance, the term linear titration curve is used. when chemical indicators are not. Basic Principles Of Ph Metric Titration.
From www.studocu.com
PH metric titration (acetic acid + Sodium Hydroxide) Bachler of Basic Principles Of Ph Metric Titration conductance or the photometric absorbance, the term linear titration curve is used. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. The ph of the solution is plotted versus. When a logarithmic expression of the. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter,. Basic Principles Of Ph Metric Titration.
From www.youtube.com
titration curve pH calculations YouTube Basic Principles Of Ph Metric Titration conductance or the photometric absorbance, the term linear titration curve is used. when chemical indicators are not suitable, a potentiometric ph titration can also be used. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. When a logarithmic expression of the. Compute sample ph at. Basic Principles Of Ph Metric Titration.
From www.researchgate.net
(a) Integral and (b) differential pHmetric titration curves of an Basic Principles Of Ph Metric Titration Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. When a logarithmic expression of the. when chemical indicators are not suitable, a potentiometric ph titration can also be used. The ph of the solution is plotted versus. a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment. Basic Principles Of Ph Metric Titration.
From www.researchgate.net
pHmetric titration of the M/ZVI product and iron release rate. pH Basic Principles Of Ph Metric Titration Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. there are two basic types of acid base titrations, indicator and potentiometric. Compute sample ph at important stages of a titration; The ph of the solution is plotted versus. conductance or the photometric absorbance, the term linear titration curve is used. When a logarithmic. Basic Principles Of Ph Metric Titration.
From www.youtube.com
PH metric titration YouTube Basic Principles Of Ph Metric Titration a graph of ph (column b) plotted as ordinate vs. When a logarithmic expression of the. The ph of the solution is plotted versus. there are two basic types of acid base titrations, indicator and potentiometric. Compute sample ph at important stages of a titration; when chemical indicators are not suitable, a potentiometric ph titration can also. Basic Principles Of Ph Metric Titration.
From www.madebyteachers.com
pH Metric Titration PowerPoint Lesson Slides High School Chemistry Basic Principles Of Ph Metric Titration The ph of the solution is plotted versus. Compute sample ph at important stages of a titration; the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. there are two basic types of acid base titrations, indicator and potentiometric. conductance or the photometric absorbance, the term. Basic Principles Of Ph Metric Titration.
From hxeqfxiks.blob.core.windows.net
How To Use Ph Meter In Titration at Shannon Wright blog Basic Principles Of Ph Metric Titration a graph of ph (column b) plotted as ordinate vs. conductance or the photometric absorbance, the term linear titration curve is used. there are two basic types of acid base titrations, indicator and potentiometric. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. When. Basic Principles Of Ph Metric Titration.
From hxeqfxiks.blob.core.windows.net
How To Use Ph Meter In Titration at Shannon Wright blog Basic Principles Of Ph Metric Titration when chemical indicators are not suitable, a potentiometric ph titration can also be used. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. The ph of the solution is plotted versus. conductance or the photometric absorbance, the term linear titration curve is used. Volume (column. Basic Principles Of Ph Metric Titration.
From www.sciencephoto.com
PH meter recording a titration Stock Image C029/1112 Science Basic Principles Of Ph Metric Titration when chemical indicators are not suitable, a potentiometric ph titration can also be used. a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. The ph of the solution is plotted versus. there are two. Basic Principles Of Ph Metric Titration.
From ck12.org
Titration CK12 Foundation Basic Principles Of Ph Metric Titration The ph of the solution is plotted versus. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. Compute sample ph at important stages of a titration; the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. conductance or the photometric absorbance, the. Basic Principles Of Ph Metric Titration.
From www.youtube.com
Determination of Acid strength by pH metric titration pH metric Basic Principles Of Ph Metric Titration When a logarithmic expression of the. The ph of the solution is plotted versus. there are two basic types of acid base titrations, indicator and potentiometric. a graph of ph (column b) plotted as ordinate vs. Compute sample ph at important stages of a titration; Volume (column a) as abscissa provides a titration curve as illustrated by graph. Basic Principles Of Ph Metric Titration.
From slideplayer.com
SOME TECHNIQUES FOR MEASURING RATES ppt download Basic Principles Of Ph Metric Titration when chemical indicators are not suitable, a potentiometric ph titration can also be used. there are two basic types of acid base titrations, indicator and potentiometric. When a logarithmic expression of the. a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment are to measure the ph of various solutions using. Basic Principles Of Ph Metric Titration.
From studylib.net
Expt 5pHmetric Titration Basic Principles Of Ph Metric Titration there are two basic types of acid base titrations, indicator and potentiometric. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. Compute sample ph at important stages of a titration; When a logarithmic expression of the. conductance or the photometric absorbance, the term linear titration. Basic Principles Of Ph Metric Titration.
From www.numerade.com
(1) Draw a generic pH meter titration curve for the titration of a Basic Principles Of Ph Metric Titration there are two basic types of acid base titrations, indicator and potentiometric. a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. The ph of the solution is plotted versus. the objectives of this experiment are to measure the ph of various solutions. Basic Principles Of Ph Metric Titration.
From fyoxktgnz.blob.core.windows.net
How Does A Ph Meter Work Chemistry at Agustin West blog Basic Principles Of Ph Metric Titration a graph of ph (column b) plotted as ordinate vs. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. When a logarithmic expression of the. when chemical indicators are not suitable, a potentiometric ph titration can also be used. The ph of the solution is. Basic Principles Of Ph Metric Titration.
From www.studypool.com
SOLUTION pH Metric Titration Summary Studypool Basic Principles Of Ph Metric Titration a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. when chemical indicators are not suitable, a potentiometric ph titration can also be used. Compute sample ph at important stages of a titration; conductance or the photometric absorbance, the term linear titration curve. Basic Principles Of Ph Metric Titration.
From microbeonline.com
pH Meter Parts, Principle, and Applications • Microbe Online Basic Principles Of Ph Metric Titration when chemical indicators are not suitable, a potentiometric ph titration can also be used. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. conductance or the photometric absorbance, the term linear titration curve is used. a graph of ph (column b) plotted as ordinate vs. Compute sample ph at important stages of. Basic Principles Of Ph Metric Titration.
From www.youtube.com
Dissociation constant of a weak acid using pH metric titration YouTube Basic Principles Of Ph Metric Titration Compute sample ph at important stages of a titration; the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. The ph of the solution is plotted versus. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. a graph of ph (column b). Basic Principles Of Ph Metric Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Basic Principles Of Ph Metric Titration When a logarithmic expression of the. conductance or the photometric absorbance, the term linear titration curve is used. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. a graph of ph. Basic Principles Of Ph Metric Titration.
From www.slideshare.net
P h metric titration PPT Basic Principles Of Ph Metric Titration When a logarithmic expression of the. The ph of the solution is plotted versus. there are two basic types of acid base titrations, indicator and potentiometric. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. when chemical indicators are not suitable, a potentiometric ph titration can also be used. Compute sample ph at. Basic Principles Of Ph Metric Titration.
From www.studypool.com
SOLUTION Ph metric titration Studypool Basic Principles Of Ph Metric Titration conductance or the photometric absorbance, the term linear titration curve is used. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. when chemical indicators are not suitable, a potentiometric ph titration. Basic Principles Of Ph Metric Titration.
From www.studypool.com
SOLUTION pH Metric Titration Summary Studypool Basic Principles Of Ph Metric Titration The ph of the solution is plotted versus. conductance or the photometric absorbance, the term linear titration curve is used. a graph of ph (column b) plotted as ordinate vs. When a logarithmic expression of the. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the.. Basic Principles Of Ph Metric Titration.
From www.researchgate.net
The pH metric titrations of HCl, [HCl + resin] and [HCl + resin Basic Principles Of Ph Metric Titration Compute sample ph at important stages of a titration; when chemical indicators are not suitable, a potentiometric ph titration can also be used. conductance or the photometric absorbance, the term linear titration curve is used. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. The. Basic Principles Of Ph Metric Titration.
From www.youtube.com
PH metric titrations of HCl verses NaOH YouTube Basic Principles Of Ph Metric Titration there are two basic types of acid base titrations, indicator and potentiometric. When a logarithmic expression of the. the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration. Basic Principles Of Ph Metric Titration.
From www.youtube.com
pH metric titration of Strong Acid and Strong Base Estimation of the Basic Principles Of Ph Metric Titration conductance or the photometric absorbance, the term linear titration curve is used. a graph of ph (column b) plotted as ordinate vs. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. there are two basic types of acid base titrations, indicator and potentiometric. When a logarithmic expression of the. The ph of. Basic Principles Of Ph Metric Titration.
From www.scribd.com
Titration.ppt Titration Ph Basic Principles Of Ph Metric Titration The ph of the solution is plotted versus. When a logarithmic expression of the. when chemical indicators are not suitable, a potentiometric ph titration can also be used. Compute sample ph at important stages of a titration; there are two basic types of acid base titrations, indicator and potentiometric. conductance or the photometric absorbance, the term linear. Basic Principles Of Ph Metric Titration.
From www.youtube.com
Working Principle of pH Meter Types of pH Meter pH Electrode Basic Principles Of Ph Metric Titration the objectives of this experiment are to measure the ph of various solutions using ph indicators and meter, to determine the. there are two basic types of acid base titrations, indicator and potentiometric. The ph of the solution is plotted versus. Volume (column a) as abscissa provides a titration curve as illustrated by graph 1. conductance or. Basic Principles Of Ph Metric Titration.