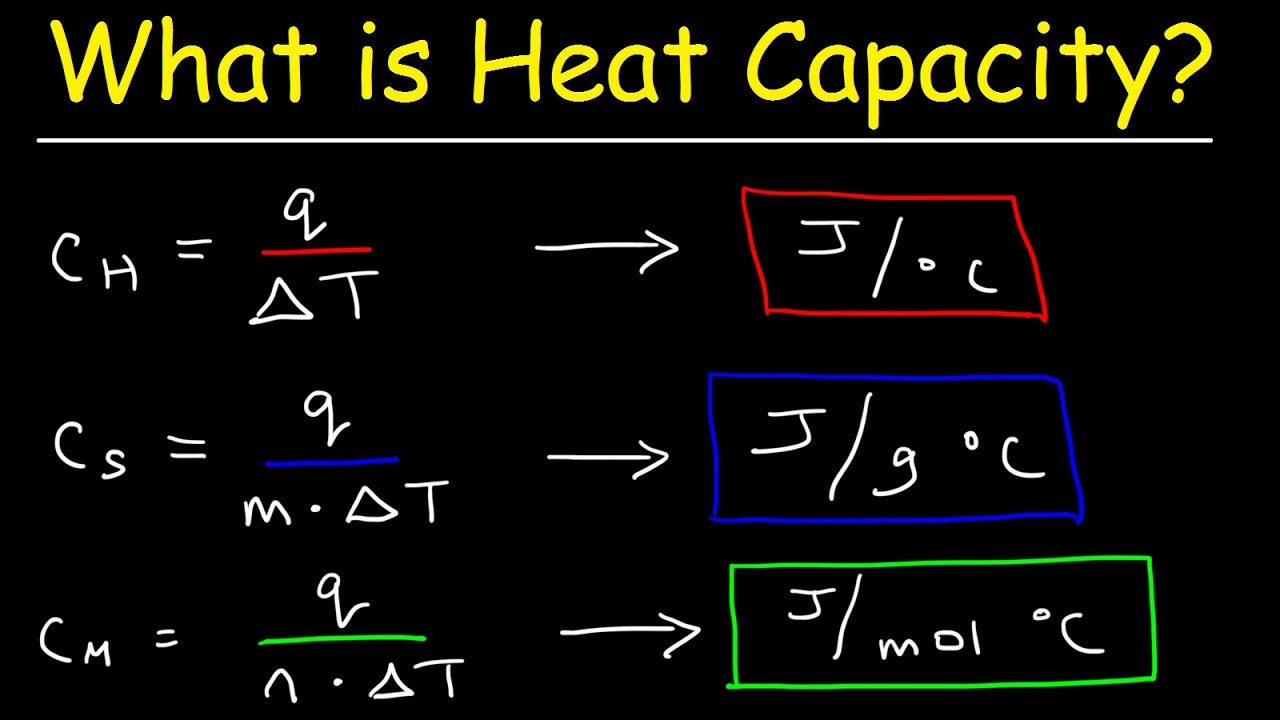
Low Vs High Specific Heat . specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). Its specific heat capacity is 4.184 j/g°c, which. water has a particularly high specific heat compared to many other substances. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. compare how quickly the different materials are heated or cooled. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. Based on these results, what material do you think has the.
from www.youtube.com
water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). compare how quickly the different materials are heated or cooled. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Based on these results, what material do you think has the.
What Is The Difference Between Specific Heat Capacity, Heat Capacity
Low Vs High Specific Heat Based on these results, what material do you think has the. water has a particularly high specific heat compared to many other substances. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which. compare how quickly the different materials are heated or cooled. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. Based on these results, what material do you think has the. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Low Vs High Specific Heat when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which. Based on these results, what material do you think has. Low Vs High Specific Heat.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Low Vs High Specific Heat Based on these results, what material do you think has the. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity. Low Vs High Specific Heat.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity Low Vs High Specific Heat Its specific heat capacity is 4.184 j/g°c, which. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. water has a particularly high specific heat compared to many other substances. the specific heat capacity is the amount of heat it takes to change the temperature of. Low Vs High Specific Heat.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Low Vs High Specific Heat the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which. Based on these results, what material do you think has the. the specific heat of a substance is the amount of energy required to raise the temperature of. Low Vs High Specific Heat.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube Low Vs High Specific Heat specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. Based on these results, what material do you think has the. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). compare how quickly the different materials are. Low Vs High Specific Heat.
From studylib.net
1.3 Specific Heat Capacity Low Vs High Specific Heat water has a particularly high specific heat compared to many other substances. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. the specific heat capacity is. Low Vs High Specific Heat.
From askanydifference.com
Specific Heat vs Heat Capacity Difference and Comparison Low Vs High Specific Heat the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which. specific heat is defined by the. Low Vs High Specific Heat.
From www.sciencefacts.net
Specific Heat and Heat Capacity Definition, Formula, Values, and Problems Low Vs High Specific Heat water has a particularly high specific heat compared to many other substances. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). the specific heat of a. Low Vs High Specific Heat.
From www.slideserve.com
PPT Heat & Specific Heat PowerPoint Presentation, free download ID Low Vs High Specific Heat Based on these results, what material do you think has the. compare how quickly the different materials are heated or cooled. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). Its specific heat capacity is 4.184 j/g°c, which. the specific heat capacity is the amount of heat it. Low Vs High Specific Heat.
From www.worksheetsplanet.com
What is Specific Heat Low Vs High Specific Heat Its specific heat capacity is 4.184 j/g°c, which. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. Based on these results, what material do you. Low Vs High Specific Heat.
From heat-transfer-thermodynamics.blogspot.com
Heat Transfer and Applied Thermodynamics Specific Heat Ratio Low Vs High Specific Heat compare how quickly the different materials are heated or cooled. Its specific heat capacity is 4.184 j/g°c, which. Based on these results, what material do you think has the. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. water has a particularly high specific heat. Low Vs High Specific Heat.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID2649066 Low Vs High Specific Heat the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). compare how quickly the different materials are heated or cooled. Its specific heat capacity is 4.184 j/g°c,. Low Vs High Specific Heat.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Low Vs High Specific Heat specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. water has a particularly high specific heat compared to many other substances. Based on these results, what material do you think has the. Its specific heat capacity is 4.184 j/g°c, which. when building calorimeters, a material. Low Vs High Specific Heat.
From www.differencebetween.com
Difference Between Heat Capacity and Specific Heat Compare the Low Vs High Specific Heat compare how quickly the different materials are heated or cooled. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. Based on these results, what material do you think has the. Its specific heat capacity is 4.184 j/g°c, which. water has a particularly high specific heat. Low Vs High Specific Heat.
From ch301.cm.utexas.edu
heating curve Low Vs High Specific Heat Its specific heat capacity is 4.184 j/g°c, which. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. the specific heat capacity is the amount. Low Vs High Specific Heat.
From phys.libretexts.org
13.2 Specific Heat Physics LibreTexts Low Vs High Specific Heat specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. water has a particularly high specific heat compared to many other substances. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. the specific. Low Vs High Specific Heat.
From edumentors.co.uk
GCSE Physics Particle model of matter Edumentors Low Vs High Specific Heat Based on these results, what material do you think has the. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. water has a particularly high specific heat. Low Vs High Specific Heat.
From www.youtube.com
Specific heat capacity (with problems and solutions) YouTube Low Vs High Specific Heat water has a particularly high specific heat compared to many other substances. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. compare how quickly the different materials are heated or cooled. specific heat is defined by the amount of heat needed to raise. Low Vs High Specific Heat.
From large.stanford.edu
Professor Robert B. Laughlin, Department of Physics, Stanford University Low Vs High Specific Heat the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of. Low Vs High Specific Heat.
From www.downwindkennels.com
Specific Heat Capacity Low Vs High Specific Heat water has a particularly high specific heat compared to many other substances. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which. Based on these results, what material do you think has the. compare how quickly the. Low Vs High Specific Heat.
From www.slideserve.com
PPT Energy and Temperature PowerPoint Presentation, free download Low Vs High Specific Heat the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Its specific heat capacity is 4.184 j/g°c, which. compare how quickly the different materials are heated or cooled. Based on these results, what material do you think has the. water has a particularly high specific. Low Vs High Specific Heat.
From www.hanlin.com
Edexcel IGCSE Physics 复习笔记 5.2.4 Specific Heat Capacity翰林国际教育 Low Vs High Specific Heat compare how quickly the different materials are heated or cooled. Based on these results, what material do you think has the. water has a particularly high specific heat compared to many other substances. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). Its specific heat capacity is 4.184. Low Vs High Specific Heat.
From universe-review.ca
Atoms Low Vs High Specific Heat when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). Its specific heat capacity is 4.184 j/g°c, which. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. the specific heat capacity is the amount of heat it. Low Vs High Specific Heat.
From www.slideshare.net
Specific Heat Low Vs High Specific Heat Its specific heat capacity is 4.184 j/g°c, which. water has a particularly high specific heat compared to many other substances. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). Based on these results, what material do you think has the. the specific heat capacity is the amount of. Low Vs High Specific Heat.
From www.slideserve.com
PPT Chapter10 Temperature and Heat PowerPoint Presentation, free Low Vs High Specific Heat Based on these results, what material do you think has the. Its specific heat capacity is 4.184 j/g°c, which. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. compare how quickly the different materials are heated or cooled. specific heat is defined by the. Low Vs High Specific Heat.
From www.youtube.com
Specific Heat and Heat Capacity YouTube Low Vs High Specific Heat the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). compare how quickly the different materials are heated or cooled. Its specific heat capacity is 4.184 j/g°c, which.. Low Vs High Specific Heat.
From rechschem.weebly.com
Specific Heat Low Vs High Specific Heat Its specific heat capacity is 4.184 j/g°c, which. water has a particularly high specific heat compared to many other substances. Based on these results, what material do you think has the. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. compare how quickly the. Low Vs High Specific Heat.
From studymind.co.uk
Heat Capacity Study Mind Low Vs High Specific Heat when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). compare how quickly the different materials are heated or cooled. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. the specific heat capacity is the amount. Low Vs High Specific Heat.
From mytutorsource.hk
Learn About Specific Heat Capacity in Less Than 10 Minutes! Low Vs High Specific Heat Its specific heat capacity is 4.184 j/g°c, which. specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. water has a particularly high specific heat compared to many other substances. compare how quickly the different materials are heated or cooled. Based on these results, what material. Low Vs High Specific Heat.
From slideplayer.com
specific heat capacity ppt download Low Vs High Specific Heat specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. water has a particularly high specific heat compared to many other substances. compare how quickly the different materials are heated or cooled. Based on these results, what material do you think has the. Its specific heat. Low Vs High Specific Heat.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity Low Vs High Specific Heat water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which. compare how quickly the different materials are heated or cooled. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. the specific heat of. Low Vs High Specific Heat.
From www.englishworksheet.my.id
Calculating Specific Heat Worksheet English Worksheet Low Vs High Specific Heat when building calorimeters, a material with a low specific heat is often used (such as styrofoam, aluminum, etc.). compare how quickly the different materials are heated or cooled. Based on these results, what material do you think has the. water has a particularly high specific heat compared to many other substances. specific heat is defined by. Low Vs High Specific Heat.
From www.slideserve.com
PPT Science TAKS Facts PowerPoint Presentation, free download ID Low Vs High Specific Heat Based on these results, what material do you think has the. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. compare how quickly the different materials are heated or cooled. specific heat is defined by the amount of heat needed to raise the temperature. Low Vs High Specific Heat.
From www.slideserve.com
PPT Specific Heat vs. Heat capacity PowerPoint Presentation, free Low Vs High Specific Heat specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. water has a particularly high specific heat compared to many other substances. the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Based on. Low Vs High Specific Heat.
From www.slideserve.com
PPT Chapter 2 The Energy Cycle PowerPoint Presentation, free download Low Vs High Specific Heat specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1. compare how quickly the different materials are heated or cooled. the specific heat of a substance is the amount of energy required to raise the temperature of 1 gram of the. the specific heat capacity. Low Vs High Specific Heat.