Standard Enthalpy Of Formation Nh4Cl Aq . the standard enthalpy of formation of any element in its standard state is zero by definition. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). For example, although oxygen can exist as ozone (o 3 ), atomic. standand enthalpies of formation & standard entropies of common compounds. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a.
from www.chegg.com
the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. standand enthalpies of formation & standard entropies of common compounds. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
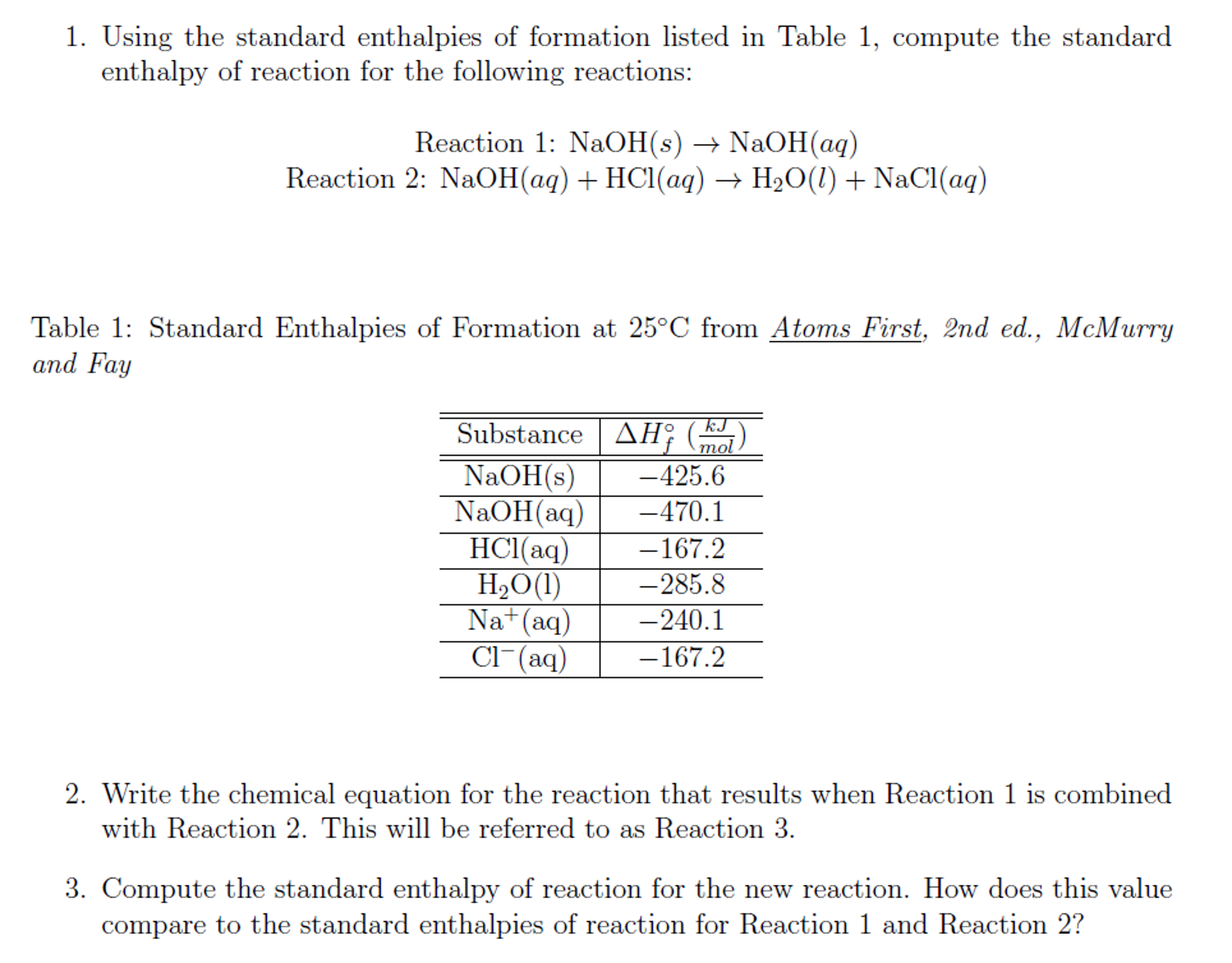
Solved Using the standard enthalpies of formation listed in
Standard Enthalpy Of Formation Nh4Cl Aq Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. standand enthalpies of formation & standard entropies of common compounds. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created.
From narodnatribuna.info
Calculating Reaction Enthalpy From Enthalpies Of Formation Standard Enthalpy Of Formation Nh4Cl Aq this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. For example, although oxygen can exist as ozone (o 3 ),. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chemistryspace.com
Standard Enthalpy of Formation Standard Enthalpy Of Formation Nh4Cl Aq this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. the standard enthalpy of formation of any element in its standard state is zero by definition. standand enthalpies of formation & standard entropies of common compounds. 136 rows standard enthalpy change of formation (data table) these tables include heat. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
DISSOLUTION OF AMMONIUM CHLORIDE NH4Cl (s) → NH4+(aq) + Cl(aq) Steps Standard Enthalpy Of Formation Nh4Cl Aq Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. For example, although oxygen can exist as ozone (o 3 ), atomic. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr). Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Enthalpy Of Formation Nh4Cl Aq standand enthalpies of formation & standard entropies of common compounds. the standard enthalpy of formation of any element in its standard state is zero by definition. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. For example,. Standard Enthalpy Of Formation Nh4Cl Aq.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Nh4Cl Aq Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. For example, although oxygen can exist as ozone (o 3 ), atomic. standand enthalpies of formation & standard entropies of common compounds. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. the standard enthalpy of formation of any. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Use the standard enthalpy of formation (ΔHf) values in Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation of any element in its standard state is zero by definition. standand enthalpies of formation & standard entropies of common compounds. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: this. Standard Enthalpy Of Formation Nh4Cl Aq.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation Nh4Cl Aq Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. top 10 species with enthalpies of formation correlated to the. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Standard Enthalpy Of Formation Nh4Cl Aq standand enthalpies of formation & standard entropies of common compounds. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. top 10 species with enthalpies of formation correlated. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved Use the data in this table of formation enthalpies to Standard Enthalpy Of Formation Nh4Cl Aq 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). Ag. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED The standard enthalpy of formation of NH4Cl(s) is 315.4 kJ/mol Standard Enthalpy Of Formation Nh4Cl Aq standand enthalpies of formation & standard entropies of common compounds. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.youtube.com
LNL Enthalpy change for the of NH4Cl YouTube Standard Enthalpy Of Formation Nh4Cl Aq 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation is a measure. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. standand enthalpies of formation & standard entropies of common compounds. top 10 species with enthalpies of formation correlated. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. standand enthalpies of formation & standard entropies of common compounds. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: . Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation Nh4Cl Aq For example, although oxygen can exist as ozone (o 3 ), atomic. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). 136 rows standard enthalpy change of formation (data table). Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved 3. You are given the following standard enthalpies of Standard Enthalpy Of Formation Nh4Cl Aq Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: standand enthalpies of formation & standard entropies of common compounds. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. . Standard Enthalpy Of Formation Nh4Cl Aq.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Nh4Cl Aq this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. standand enthalpies of formation & standard entropies of common compounds. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). the standard enthalpy of formation of any element in its. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Enthalpy Of Formation Nh4Cl Aq For example, although oxygen can exist as ozone (o 3 ), atomic. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Ag s 0 42.6 ag+ aq 105.79 72.7. Standard Enthalpy Of Formation Nh4Cl Aq.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Nh4Cl Aq 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). For example, although oxygen can exist as ozone (o 3 ), atomic. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. the standard enthalpy of formation of any element in. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED A scientist measures the standard enthalpy change for the Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation of any element in its standard state is zero by definition. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: For example, although oxygen can exist as ozone (o 3 ), atomic. standand enthalpies of formation & standard entropies of common compounds. Ag. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: this calculator uses the enthalpy of formation of the compounds to. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Nh4Cl Aq 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. For example,. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Nh4Cl Aq standand enthalpies of formation & standard entropies of common compounds. the standard enthalpy of formation of any element in its standard state is zero by definition. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Standard Enthalpy Of Formation Nh4Cl Aq standand enthalpies of formation & standard entropies of common compounds. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). the standard enthalpy of formation of any element in. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved The standard enthalpy of formation of NH4Cl(s) is Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). standand enthalpies of formation &. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Question 3 (0.5 points) Select the best net ionic equation for Standard Enthalpy Of Formation Nh4Cl Aq this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. the standard enthalpy of formation of any element in its standard state is zero by definition. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. For example,. Standard Enthalpy Of Formation Nh4Cl Aq.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). the standard enthalpy of formation. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Nh4Cl Aq the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation of any element in its standard state is zero by definition. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a.. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved You are given the following standard enthalpies of Standard Enthalpy Of Formation Nh4Cl Aq 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: For example, although oxygen can exist as ozone (o 3 ), atomic. the standard enthalpy of formation of any element. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Given the following reactions, what is the overall enthalpy Standard Enthalpy Of Formation Nh4Cl Aq 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. the standard enthalpy of formation. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Nh4Cl Aq For example, although oxygen can exist as ozone (o 3 ), atomic. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation of any element in its standard state is zero by definition.. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Enthalpy Of Formation Nh4Cl Aq 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation of any element in its standard state is zero by definition. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). Ag s 0. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Nh4Cl Aq standand enthalpies of formation & standard entropies of common compounds. the standard enthalpy of formation of any element in its standard state is zero by definition. top 10 species with enthalpies of formation correlated to the δ f h° of (nh4)cl (cr) please note: 136 rows standard enthalpy change of formation (data table) these tables include. Standard Enthalpy Of Formation Nh4Cl Aq.
From www.numerade.com
SOLVED Using standard heats of formation, calculate the standard Standard Enthalpy Of Formation Nh4Cl Aq Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. standand enthalpies of formation & standard entropies of common compounds. the standard enthalpy of formation of any element in its standard state is zero by definition. this calculator uses the enthalpy of formation of the compounds to calculate the enthalpy change from a. the standard. Standard Enthalpy Of Formation Nh4Cl Aq.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Nh4Cl Aq 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Ag s 0 42.6 ag+ aq 105.79 72.7 agcl s −127.01. 21 rows the 20 contributors listed below account only for 87.3% of the provenance of δfh° of (nh4)cl (cr). this calculator uses the enthalpy of formation of. Standard Enthalpy Of Formation Nh4Cl Aq.