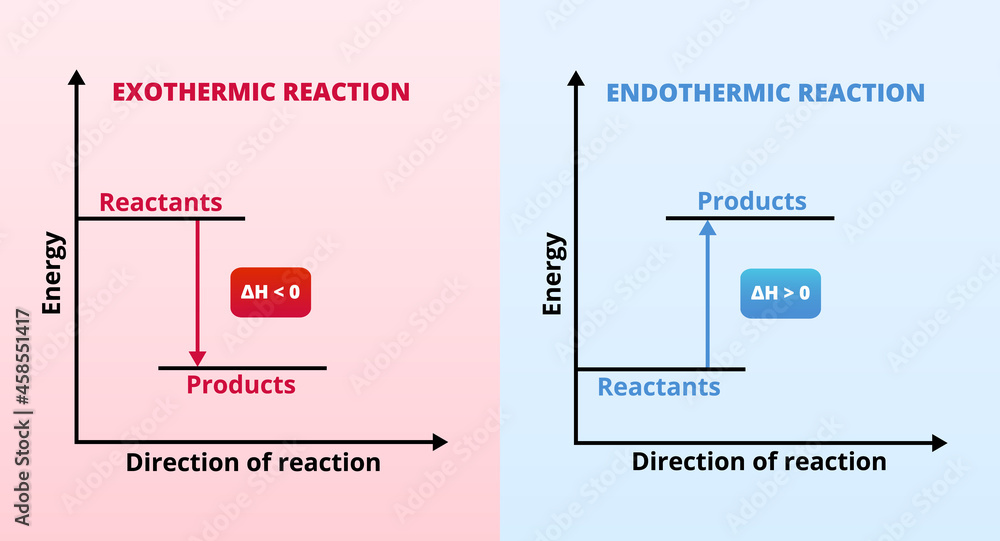
Endothermic Delta H . The following conventions apply when using δh: In chemical reactions, bond breaking. A positive δh means that heat flows into a. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A negative δh means that heat flows from a system to its surroundings; Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. Learn about enthalpy and the expected change in exothermic and endothermic reactions. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. Define what constitutes an exothermic and endothermic chemical reaction.
from stock.adobe.com
Learn about enthalpy and the expected change in exothermic and endothermic reactions. A negative δh means that heat flows from a system to its surroundings; An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. The following conventions apply when using δh: Define what constitutes an exothermic and endothermic chemical reaction. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In chemical reactions, bond breaking.
Vecteur Stock Vector graphs or charts of endothermic and exothermic
Endothermic Delta H In chemical reactions, bond breaking. A positive δh means that heat flows into a. Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. Learn about enthalpy and the expected change in exothermic and endothermic reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In chemical reactions, bond breaking. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. A negative δh means that heat flows from a system to its surroundings; The following conventions apply when using δh: Define what constitutes an exothermic and endothermic chemical reaction. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic.
From www.ck12.org
Potential Energy Diagrams ( Read ) Chemistry CK12 Foundation Endothermic Delta H A positive δh means that heat flows into a. A negative δh means that heat flows from a system to its surroundings; Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the. Endothermic Delta H.
From www.haikudeck.com
Thermochemistry Key Concepts by avasquez704170 Endothermic Delta H Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. A negative δh means that heat flows from a system to its surroundings; A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Define what constitutes an exothermic and endothermic chemical reaction. A chemical reaction that has a positive δh. Endothermic Delta H.
From www.youtube.com
5.1.1 (New Syll 5.3) Exothermic, Endothermic, Delta H Energy Profiles Endothermic Delta H Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; In chemical reactions, bond breaking. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. A positive. Endothermic Delta H.
From quizlet.com
Calculate \Delta H^\circ for the following reaction using Quizlet Endothermic Delta H In chemical reactions, bond breaking. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Define what constitutes. Endothermic Delta H.
From slideplayer.com
and Equilibrium ppt download Endothermic Delta H A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. Learn. Endothermic Delta H.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Delta H A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; The following conventions apply when using δh: Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. Learn about enthalpy and the expected change in exothermic and endothermic reactions. In chemical reactions, bond breaking. Define what constitutes an exothermic and. Endothermic Delta H.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Delta H In chemical reactions, bond breaking. Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; A negative δh means that heat flows from a system to its surroundings; The following conventions apply when using δh: Learn about enthalpy and the. Endothermic Delta H.
From cherries-everwhere.blogspot.com
Delta H / Solving For Delta H Of Formation 1 Byu Idaho cherrieseverwhere Endothermic Delta H A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; A positive δh means that heat flows into a. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A negative δh means that heat flows from a system to its. Endothermic Delta H.
From www.vedantu.com
For an endothermic reaction where {\\bf{\\Delta H}} represents the Endothermic Delta H The following conventions apply when using δh: A positive δh means that heat flows into a. Learn about enthalpy and the expected change in exothermic and endothermic reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. A chemical reaction that has a positive δh is said to. Endothermic Delta H.
From igcsechemistryrevision.weebly.com
iGCSE CHEMISTRY REVISION HELP Acids & Energetics Endothermic Delta H Define what constitutes an exothermic and endothermic chemical reaction. A negative δh means that heat flows from a system to its surroundings; Learn about enthalpy and the expected change in exothermic and endothermic reactions. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. An endothermic reaction is a chemical change in. Endothermic Delta H.
From www.doubtnut.com
For an endothermic reaction, where Delta H represents the enthalpy of Endothermic Delta H Define what constitutes an exothermic and endothermic chemical reaction. Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. The following conventions apply when using δh: A chemical reaction. Endothermic Delta H.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1846781 Endothermic Delta H An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. The following conventions apply when using δh: Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. A negative value of an enthalpy change, δh < 0, indicates an. Endothermic Delta H.
From www.youtube.com
Change in enthalpy can be positive or negative Reactions meriSTEM Endothermic Delta H Learn about enthalpy and the expected change in exothermic and endothermic reactions. In chemical reactions, bond breaking. A positive δh means that heat flows into a. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Endothermic and exothermic reactions can be thought of as. Endothermic Delta H.
From www.doubtnut.com
[Odia] For an endothermic reaction where, Delta H represent the enthal Endothermic Delta H A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Given prior knowledge of the thermodynamic terms. Endothermic Delta H.
From www.doubtnut.com
For an endothermic reaction, where Delta H represents the enthalpy of Endothermic Delta H In chemical reactions, bond breaking. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Thus δh rxn < 0 for an exothermic reaction,. Endothermic Delta H.
From www.scribd.com
Endothermic Reaction Exothermic Reaction Delta H Delta H PDF Endothermic Delta H Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. A positive δh means that heat flows into a. In chemical reactions, bond breaking. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. Given prior knowledge of the thermodynamic terms. Endothermic Delta H.
From edurev.in
For an endothermic reaction energy of activation is EA and enthalpy of Endothermic Delta H In chemical reactions, bond breaking. Define what constitutes an exothermic and endothermic chemical reaction. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Learn about enthalpy and the expected change in exothermic and endothermic reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a. Endothermic Delta H.
From www.gettyimages.co.uk
Endothermic And Exothermic Chemical Reactions Two Chemical Reactions Endothermic Delta H A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. Learn about enthalpy and the expected change in exothermic and endothermic reactions. A positive δh means that heat flows into a. The following conventions apply when using δh: Endothermic and exothermic reactions can. Endothermic Delta H.
From slideplayer.com
Period 3 Advanced Chemistry ppt download Endothermic Delta H A positive δh means that heat flows into a. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Learn about enthalpy and the expected change in exothermic and endothermic reactions. A chemical reaction that has a positive. Endothermic Delta H.
From mungfali.com
Exothermic Vs Endothermic Diagram Endothermic Delta H Define what constitutes an exothermic and endothermic chemical reaction. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. A positive δh means that heat flows into a. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for. Endothermic Delta H.
From general.chemistrysteps.com
The Effect of 𝚫H, 𝚫S, and T on 𝚫G Spontaneity Chemistry Steps Endothermic Delta H A positive δh means that heat flows into a. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its. Endothermic Delta H.
From www.chegg.com
Solved Given the following values of Delta H and Delta S for Endothermic Delta H In chemical reactions, bond breaking. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; A positive δh means that heat flows into a. Define what constitutes an exothermic and endothermic. Endothermic Delta H.
From www.youtube.com
For an endothermic reaction, where `Delta H` represents the enthalpy of Endothermic Delta H A negative δh means that heat flows from a system to its surroundings; The following conventions apply when using δh: Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase. Endothermic Delta H.
From www.numerade.com
SOLVED Explain the difference between an exothermic and endothermic Endothermic Delta H In chemical reactions, bond breaking. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a.. Endothermic Delta H.
From www.studyorgo.com
Organic Chemistry Help Tools Endothermic Delta H An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. A positive δh means that heat flows into a. Define what constitutes an exothermic and endothermic chemical reaction. Learn. Endothermic Delta H.
From www.youtube.com
For an endothermic reaction energy of activation is `E_(a)` and enthlpy Endothermic Delta H A negative δh means that heat flows from a system to its surroundings; The following conventions apply when using δh: In chemical reactions, bond breaking. A positive δh means that heat flows into a. Define what constitutes an exothermic and endothermic chemical reaction. A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction. Endothermic Delta H.
From mungfali.com
Endothermic Diagram With Labels Endothermic Delta H Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. The following conventions apply when using δh: Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In chemical reactions, bond breaking. Define what constitutes an exothermic and endothermic chemical reaction.. Endothermic Delta H.
From www.pinterest.ph
ExothermicEndothermic Processes Chemistry Help, Organic Chemistry Endothermic Delta H A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. Define what constitutes an exothermic and endothermic. Endothermic Delta H.
From fixdiagramzoolatrous.z21.web.core.windows.net
Enthalpy Diagram For Endothermic Reaction Endothermic Delta H Define what constitutes an exothermic and endothermic chemical reaction. Learn about enthalpy and the expected change in exothermic and endothermic reactions. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction.. Endothermic Delta H.
From www.chegg.com
Solved Identify each process as endothermic or exothermic, Endothermic Delta H Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; In chemical reactions, bond breaking. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic reaction. An endothermic reaction is a chemical change. Endothermic Delta H.
From www.slideserve.com
PPT Enthalpy (H) and Change in Enthalpy ( Δ H ) PowerPoint Endothermic Delta H In chemical reactions, bond breaking. A positive δh means that heat flows into a. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Learn about enthalpy and the expected change in exothermic and endothermic reactions. A negative δh means that heat flows from a system to its surroundings;. Endothermic Delta H.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Delta H Given prior knowledge of the thermodynamic terms entropy, enthalpy, and spontaneous processes, students will gain a. A positive δh means that heat flows into a. A negative value of an enthalpy change, δh < 0, indicates an exothermic reaction; Learn about enthalpy and the expected change in exothermic and endothermic reactions. A negative δh means that heat flows from a. Endothermic Delta H.
From www.youtube.com
01. Delta H determination Exothermic and Endothermic Chemistry and Endothermic Delta H Define what constitutes an exothermic and endothermic chemical reaction. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A positive δh means that heat flows into a. Thus δh rxn < 0 for an exothermic reaction, and δh rxn > 0 for an endothermic. Endothermic Delta H.
From stock.adobe.com
Vecteur Stock Vector graphs or charts of endothermic and exothermic Endothermic Delta H A negative δh means that heat flows from a system to its surroundings; A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. A positive δh means that heat flows into a. Learn about enthalpy and the expected change in exothermic and endothermic. Endothermic Delta H.
From www.numerade.com
SOLVED a. is this an exothermic or endothermic reaction? b. what is Endothermic Delta H A chemical reaction that has a positive δh is said to be endothermic, while a chemical reaction that has a negative δh is said to be exothermic. Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. In chemical reactions, bond breaking. Define what constitutes an exothermic and endothermic. Endothermic Delta H.