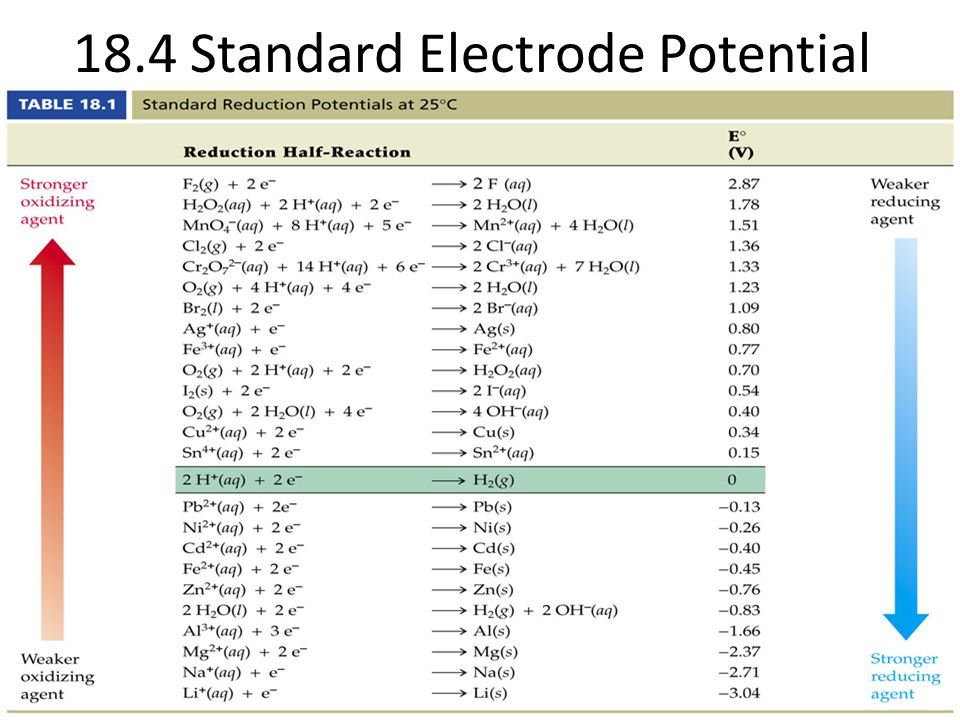
Standard Reduction Potential Ethanol . One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. Standard reduction potentials by value. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides eo for selected reduction reactions. You may rewrite a reaction by replacing h + with h 3 o + and. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to.
from ar.inspiredpencil.com
(reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potentials by value. The following table provides eo for selected reduction reactions. You may rewrite a reaction by replacing h + with h 3 o + and. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Reduction reactions in acidic solution are written using h + in place of h 3 o +.
Standard Reduction Potential Table
Standard Reduction Potential Ethanol The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. The following table provides eo for selected reduction reactions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. Standard reduction potentials by value. You may rewrite a reaction by replacing h + with h 3 o + and.
From www.chegg.com
Solved A. Calculate DeltaG' For The Oxidation Of Aketogl... Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. Standard reduction potentials by value. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Reduction reactions in acidic solution are written using h + in place. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved Standard Reduction Potentials, E'o Halfreaction Standard Reduction Potential Ethanol If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The following table provides eo for selected reduction reactions. Reduction reactions in acidic solution. Standard Reduction Potential Ethanol.
From www.tpsearchtool.com
Standard Oxidation Reduction Potentials Free Energy Values According To Standard Reduction Potential Ethanol The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Standard reduction potentials by value. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. You may rewrite a reaction by replacing h + with h 3 o +. Standard Reduction Potential Ethanol.
From dxoqcxjmj.blob.core.windows.net
Standard Reduction Potential Example at Toni Officer blog Standard Reduction Potential Ethanol The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The. Standard Reduction Potential Ethanol.
From www.researchgate.net
Gibbs free energy changes and standard reduction potentials at 25 • C Standard Reduction Potential Ethanol The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Standard reduction potentials by value. If we examine a table of standard reduction potential, we see under standard conditions that a. Standard Reduction Potential Ethanol.
From dxoasuand.blob.core.windows.net
Calculate The Standard Cell Potentials Given The Following Data at Standard Reduction Potential Ethanol The following table provides eo for selected reduction reactions. Standard reduction potentials by value. Reduction reactions in acidic solution are written using h + in place of h 3 o +. You may rewrite a reaction by replacing h + with h 3 o + and. The standard reduction potential can be determined by subtracting the standard reduction potential for. Standard Reduction Potential Ethanol.
From loensgcfn.blob.core.windows.net
Standard Reduction Potential Calculator at James Burkley blog Standard Reduction Potential Ethanol The following table provides eo for selected reduction reactions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential is the tendency for a chemical species to be reduced, and. Standard Reduction Potential Ethanol.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Ethanol (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The following table provides eo for selected reduction reactions. Standard reduction potentials by value. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. One way to quantify whether. Standard Reduction Potential Ethanol.
From www.numerade.com
SOLVED TABLE 137b Standard Reduction Potentials of Some Biologically Standard Reduction Potential Ethanol Standard reduction potentials by value. The following table provides eo for selected reduction reactions. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. You may rewrite a reaction by. Standard Reduction Potential Ethanol.
From www.numerade.com
SOLVED Selective Reduction The standard reduction potential for the Standard Reduction Potential Ethanol (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. Reduction reactions in acidic solution are written using h + in place of h 3 o +. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to.. Standard Reduction Potential Ethanol.
From www.pinterest.co.kr
pe=log[e] measuved in volts.if pe is negative reduced, if pe is Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. Standard reduction potentials by value. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. The standard reduction potential is the tendency for a chemical. Standard Reduction Potential Ethanol.
From www.numerade.com
SOLVED Standard Reduction Potentials at 25^∘C^* Copyright (c) The Standard Reduction Potential Ethanol Reduction reactions in acidic solution are written using h + in place of h 3 o +. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh. Standard Reduction Potential Ethanol.
From pdfprof.com
acetic acid to methane equation Standard Reduction Potential Ethanol (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The following table provides eo for selected reduction reactions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Standard reduction potentials by value. The standard reduction potential can be determined by subtracting the. Standard Reduction Potential Ethanol.
From www.researchgate.net
CO2 reduction potentials vs. SHE a . Download Scientific Diagram Standard Reduction Potential Ethanol You may rewrite a reaction by replacing h + with h 3 o + and. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate. Standard Reduction Potential Ethanol.
From www.ajevonline.org
Review of Oxidative Processes in Wine and Value of Reduction Potentials Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. You may. Standard Reduction Potential Ethanol.
From www.mdpi.com
Catalysts Free FullText Photocatalytic CO2 Conversion to Ethanol Standard Reduction Potential Ethanol (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. The. Standard Reduction Potential Ethanol.
From www.researchgate.net
Standard electrochemical potentials for CO 2 reduction. Download Standard Reduction Potential Ethanol Reduction reactions in acidic solution are written using h + in place of h 3 o +. The following table provides eo for selected reduction reactions. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. Standard reduction potentials by value. The standard reduction potential is the tendency for. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved TABLE 20.1 Standard Reduction Potentials for Several Standard Reduction Potential Ethanol Standard reduction potentials by value. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. You may rewrite a reaction by replacing h + with h 3 o + and. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction. Standard Reduction Potential Ethanol.
From www.scribd.com
Table of Standard Reduction Potential PDF Standard Reduction Potential Ethanol Reduction reactions in acidic solution are written using h + in place of h 3 o +. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved Standard Redox Potentials of Selected HalfReactions Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. The following table provides eo for selected reduction reactions. You may rewrite a reaction by replacing h + with h 3 o + and. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved Table 18.1 Standard reduction potentials of some Standard Reduction Potential Ethanol Standard reduction potentials by value. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. The following table provides eo for selected reduction reactions. You may rewrite a reaction by replacing h + with h 3 o + and. If we examine a table of standard reduction potential, we. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved Table 20.1 Standard Reduction Potentials for Several Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. The following table provides eo for selected reduction reactions. Reduction reactions in acidic solution are written using h + in place of h 3 o +. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of. Standard Reduction Potential Ethanol.
From exovxcvqo.blob.core.windows.net
Standard Reduction Potential Gold at Juan Delk blog Standard Reduction Potential Ethanol You may rewrite a reaction by replacing h + with h 3 o + and. Standard reduction potentials by value. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved Using standard reduction potentials from the ALEKS Standard Reduction Potential Ethanol You may rewrite a reaction by replacing h + with h 3 o + and. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The following table provides eo for selected reduction. Standard Reduction Potential Ethanol.
From slideplayer.com
Electrochemistry Part III Reduction Potentials ppt download Standard Reduction Potential Ethanol Reduction reactions in acidic solution are written using h + in place of h 3 o +. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Standard reduction potentials by. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved Use the standard reduction potentials table as needed Standard Reduction Potential Ethanol The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Reduction reactions in acidic solution are written using h + in place of h 3 o +. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. You. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved TABLE 14.1 "Electron Tower" of Standard Reduction Standard Reduction Potential Ethanol The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. You may rewrite a reaction by replacing h + with h 3 o + and. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. The following. Standard Reduction Potential Ethanol.
From www.solutioninn.com
[Solved] Use the table of standard reduction poten SolutionInn Standard Reduction Potential Ethanol Reduction reactions in acidic solution are written using h + in place of h 3 o +. The standard reduction potential is the tendency for a chemical species to be reduced, and is measured in volts at standard conditions. (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. One way. Standard Reduction Potential Ethanol.
From www.researchgate.net
(PDF) Advanced Oxidation Processes for the Removal of Antibiotics from Standard Reduction Potential Ethanol If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. Standard reduction potentials by value. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. The following table provides eo for selected reduction reactions. One way to quantify. Standard Reduction Potential Ethanol.
From www.numerade.com
SOLVEDTABLE 20.1 Standard Reduction Potentials in Water at 25'C Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. Reduction reactions in acidic solution are written using h + in place of h 3 o +. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to. Standard Reduction Potential Ethanol.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Ethanol The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Reduction reactions in acidic solution are written using h + in place of h 3 o +. One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. (reduction of oxygen, e.g.,. Standard Reduction Potential Ethanol.
From ar.inspiredpencil.com
Standard Reduction Potential Table Standard Reduction Potential Ethanol (reduction of oxygen, e.g., respiration, yields more energy than denitrification, while production of oxygen from water, e.g., photosynthesis, takes. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate. Standard Reduction Potential Ethanol.
From www.chegg.com
Solved What is the standard reduction potential (x) for the Standard Reduction Potential Ethanol You may rewrite a reaction by replacing h + with h 3 o + and. The standard reduction potential can be determined by subtracting the standard reduction potential for the reaction occurring at. Reduction reactions in acidic solution are written using h + in place of h 3 o +. The standard reduction potential is the tendency for a chemical. Standard Reduction Potential Ethanol.
From klaecwhyz.blob.core.windows.net
Standard Reduction Potential Ph at Shane Marlowe blog Standard Reduction Potential Ethanol If we examine a table of standard reduction potential, we see under standard conditions that a transfer of electrons from nadh to pyruvate to. Reduction reactions in acidic solution are written using h + in place of h 3 o +. Standard reduction potentials by value. The standard reduction potential can be determined by subtracting the standard reduction potential for. Standard Reduction Potential Ethanol.
From www.researchgate.net
Standard reduction potentials versus SHE; data from references [89,91 Standard Reduction Potential Ethanol One way to quantify whether a substance is a strong oxidizing agent or a strong reducing agent is to use the. Standard reduction potentials by value. Reduction reactions in acidic solution are written using h + in place of h 3 o +. If we examine a table of standard reduction potential, we see under standard conditions that a transfer. Standard Reduction Potential Ethanol.