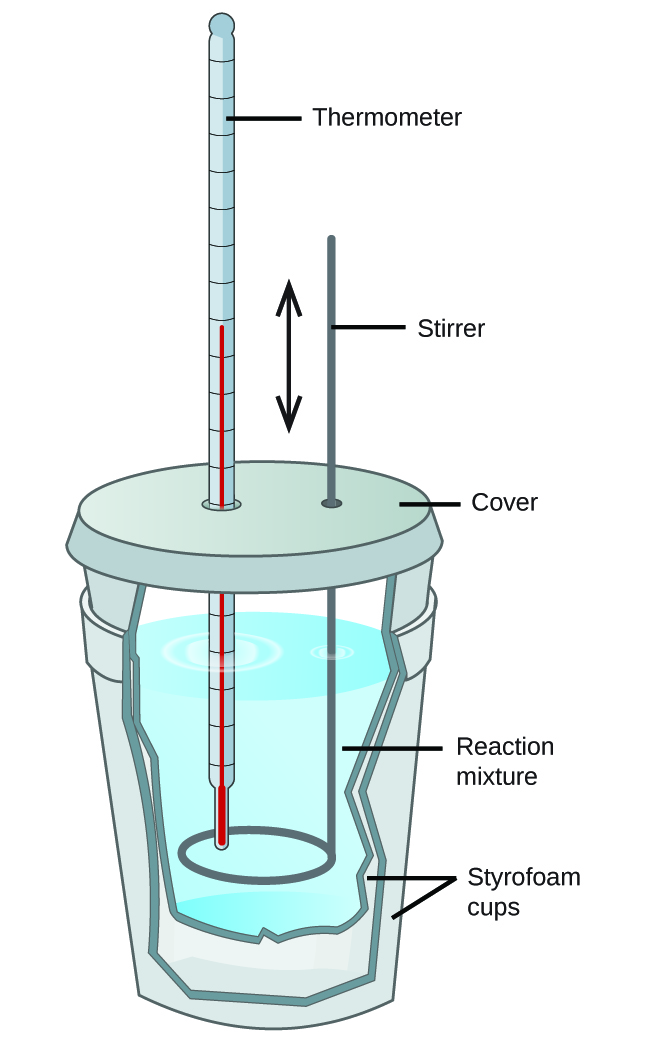
Calorimetry Lab Setup . As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Heat is a form of energy that is transferred between objects with different temperatures. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. By the end of this section, you will be able to: Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Calculate and interpret heat and. In this lab, you will do two classic calorimetry experiments:
from ecampusontario.pressbooks.pub
Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Calculate and interpret heat and. Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. In this lab, you will do two classic calorimetry experiments: Calculate and interpret heat and related properties using typical calorimetry data. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As part of this lab, you will:
5.2 Calorimetry Chemistry
Calorimetry Lab Setup In this lab, you will do two classic calorimetry experiments: Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Calculate and interpret heat and related properties using typical calorimetry data. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In this lab, you will do two classic calorimetry experiments: Explain the technique of calorimetry. As part of this lab, you will: Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. By the end of this section, you will be able to: Heat is a form of energy that is transferred between objects with different temperatures. Explain the technique of calorimetry. Calculate and interpret heat and.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Lab Setup Calculate and interpret heat and. As part of this lab, you will: Heat is a form of energy that is transferred between objects with different temperatures. Explain the technique of calorimetry. In this lab, you will do two classic calorimetry experiments: Calculate and interpret heat and related properties using typical calorimetry data. Students will work in pairs with one person. Calorimetry Lab Setup.
From www.youtube.com
Calorimetry Part I Lab Version) YouTube Calorimetry Lab Setup In this lab, you will do two classic calorimetry experiments: Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As part of this lab, you will:. Calorimetry Lab Setup.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Lab Setup By the end of this section, you will be able to: Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Heat is a form of energy that is transferred between objects with different temperatures. Explain the technique of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals.. Calorimetry Lab Setup.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Lab Setup Heat is a form of energy that is transferred between objects with different temperatures. Explain the technique of calorimetry. In this lab, you will do two classic calorimetry experiments: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. By the end of this section, you will be. Calorimetry Lab Setup.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Lab Setup Calculate and interpret heat and. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Explain the technique of calorimetry. By the end of this section, you will be able. Calorimetry Lab Setup.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Lab Setup In this lab, you will do two classic calorimetry experiments: Heat is a form of energy that is transferred between objects with different temperatures. As part of this lab, you will: 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. By the end of this section, you will be able to: Explain the technique of calorimetry. Calculate and interpret. Calorimetry Lab Setup.
From www.youtube.com
How to Draw a Calorimeter Step by Step Drawing Tutorial YouTube Calorimetry Lab Setup Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Explain the technique of calorimetry. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calculate and interpret heat and. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides. Calorimetry Lab Setup.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetry Lab Setup Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Heat is a form of energy that is transferred between objects with different temperatures. By the end of this section, you will be able to: Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Measuring the latent. Calorimetry Lab Setup.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Setup As part of this lab, you will: Calculate and interpret heat and. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner. Calorimetry Lab Setup.
From www.youtube.com
Calorimetry Lab Video YouTube Calorimetry Lab Setup Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and. As part of this lab, you will: Explain the technique of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Calorimetry is. Calorimetry Lab Setup.
From saylordotorg.github.io
Calorimetry Calorimetry Lab Setup In this lab, you will do two classic calorimetry experiments: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calculate and interpret heat and related properties using typical calorimetry data. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Heat is a form of energy that. Calorimetry Lab Setup.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Lab Setup Calculate and interpret heat and. Explain the technique of calorimetry. Explain the technique of calorimetry. Calculate and interpret heat and related properties using typical calorimetry data. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. By the end of this section, you will be able to: As. Calorimetry Lab Setup.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Lab Setup Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Calculate and interpret heat and related properties using typical calorimetry data. Explain the technique of calorimetry. As part of this lab, you will: 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. In this lab, you will do two. Calorimetry Lab Setup.
From www.youtube.com
Calorimetry Virtual Lab YouTube Calorimetry Lab Setup Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. In this lab, you will do two classic calorimetry experiments: 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Calculate and interpret heat and. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of. Calorimetry Lab Setup.
From www.youtube.com
Unit 8 Food Calorimetry Lab YouTube Calorimetry Lab Setup Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Explain the technique of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Explain the technique of calorimetry. By the end of this section, you will be able to: As part of this lab, you will: Calculate and. Calorimetry Lab Setup.
From wongchemistry.weebly.com
Food Calorimetry Lab WongChemistry Calorimetry Lab Setup Calculate and interpret heat and. In this lab, you will do two classic calorimetry experiments: Explain the technique of calorimetry. As part of this lab, you will: Explain the technique of calorimetry. By the end of this section, you will be able to: Heat is a form of energy that is transferred between objects with different temperatures. Calorimetry is the. Calorimetry Lab Setup.
From www.researchgate.net
Differential Scanning Calorimetry (DSC) Setup Download Scientific Diagram Calorimetry Lab Setup Heat is a form of energy that is transferred between objects with different temperatures. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calculate and interpret heat and related properties using typical calorimetry data. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. 6‐3a. Calorimetry Lab Setup.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Setup As part of this lab, you will: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Explain the technique of calorimetry. Heat. Calorimetry Lab Setup.
From www.researchgate.net
Illustration of the calorimetry testing fixture. The setup is designed Calorimetry Lab Setup In this lab, you will do two classic calorimetry experiments: Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Calculate and. Calorimetry Lab Setup.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Lab Setup Calculate and interpret heat and. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Explain the technique of calorimetry. In this lab, you will do two classic calorimetry experiments: Heat is a form of energy that is transferred between objects with different temperatures. Students will work in pairs with one person swirling the cup and reading the. Calorimetry Lab Setup.
From www.youtube.com
FE11 VCE Unit 3 Calorimetry YouTube Calorimetry Lab Setup Explain the technique of calorimetry. Heat is a form of energy that is transferred between objects with different temperatures. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Calculate and interpret heat and. Explain the technique of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. By. Calorimetry Lab Setup.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Lab Setup Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. In this lab, you will do two classic calorimetry experiments: Heat is a form of energy that is transferred between objects with different temperatures. By the end of this section, you will be able to: Students will work in pairs with one person swirling the cup and reading. Calorimetry Lab Setup.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Setup Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. By the end of this section, you will be able to: Explain the technique of calorimetry. As part of this lab, you will: In this lab, you will. Calorimetry Lab Setup.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Lab Setup Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides of eqn. Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. By. Calorimetry Lab Setup.
From maestrovirtuale.com
Calorimetria o que você estuda e aplicações Calorimetry Lab Setup Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Heat is a form of energy that is transferred between objects with different temperatures. Explain the technique of calorimetry. Explain the technique of calorimetry. Calculate and interpret heat and. By the end of this section, you will be able to:. Calorimetry Lab Setup.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Calorimetry Lab Setup In this lab, you will do two classic calorimetry experiments: Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Explain the technique of calorimetry. Calculate and interpret heat and. Explain the technique of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of. Calorimetry Lab Setup.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Lab Setup Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In this lab, you will do two classic calorimetry experiments: Explain the technique of calorimetry. Experiment 6 ∙ calorimetry 6‐5. Calorimetry Lab Setup.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Lab Setup As part of this lab, you will: Calculate and interpret heat and related properties using typical calorimetry data. Calculate and interpret heat and. Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Calorimetry is the study of heat transferred in a chemical reaction, and. Calorimetry Lab Setup.
From www.youtube.com
Calorimetry Lab 2 YouTube Calorimetry Lab Setup 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Explain the technique of calorimetry. In this lab, you will do two classic calorimetry experiments: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is. Calorimetry Lab Setup.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry Calorimetry Lab Setup Calculate and interpret heat and. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Explain the technique of calorimetry. 6‐3a by nsys and making the substi‐ tutions. Calorimetry Lab Setup.
From www.flickr.com
Calorimetry Lab Kentucky Country Day Flickr Calorimetry Lab Setup Explain the technique of calorimetry. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. In this lab, you will do two classic calorimetry experiments: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is. Calorimetry Lab Setup.
From saylordotorg.github.io
Calorimetry Calorimetry Lab Setup By the end of this section, you will be able to: Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Explain the technique of calorimetry. In this lab, you will do two classic calorimetry experiments: Experiment 6 ∙ calorimetry. Calorimetry Lab Setup.
From www.youtube.com
Calorimetry Part II Lab Version) YouTube Calorimetry Lab Setup Explain the technique of calorimetry. 6‐3a by nsys and making the substi‐ tutions δhsys/nsys δh. Explain the technique of calorimetry. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As part of this lab, you will: Calculate and interpret heat and. Students will work in pairs with one person swirling the. Calorimetry Lab Setup.
From www.blitznotes.org
Chemical Energetics Calorimetry Lab Setup By the end of this section, you will be able to: Heat is a form of energy that is transferred between objects with different temperatures. Students will work in pairs with one person swirling the cup and reading the thermometer, while their partner keeps time. Explain the technique of calorimetry. Experiment 6 ∙ calorimetry 6‐5 n =m/m dividing both sides. Calorimetry Lab Setup.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Lab Setup Heat is a form of energy that is transferred between objects with different temperatures. Explain the technique of calorimetry. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. As part of this lab, you will: Measuring the latent heat of fusion of water, and measuring the specific. Calorimetry Lab Setup.