Acetic Acid Buffer Equation . An example of a buffer that consists of a weak. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. It is a buffer because it contains both. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Consider the dissociation equation for acetic acid: Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back.
from www.shutterstock.com
Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. It is a buffer because it contains both. An example of a buffer that consists of a weak. Consider the dissociation equation for acetic acid:
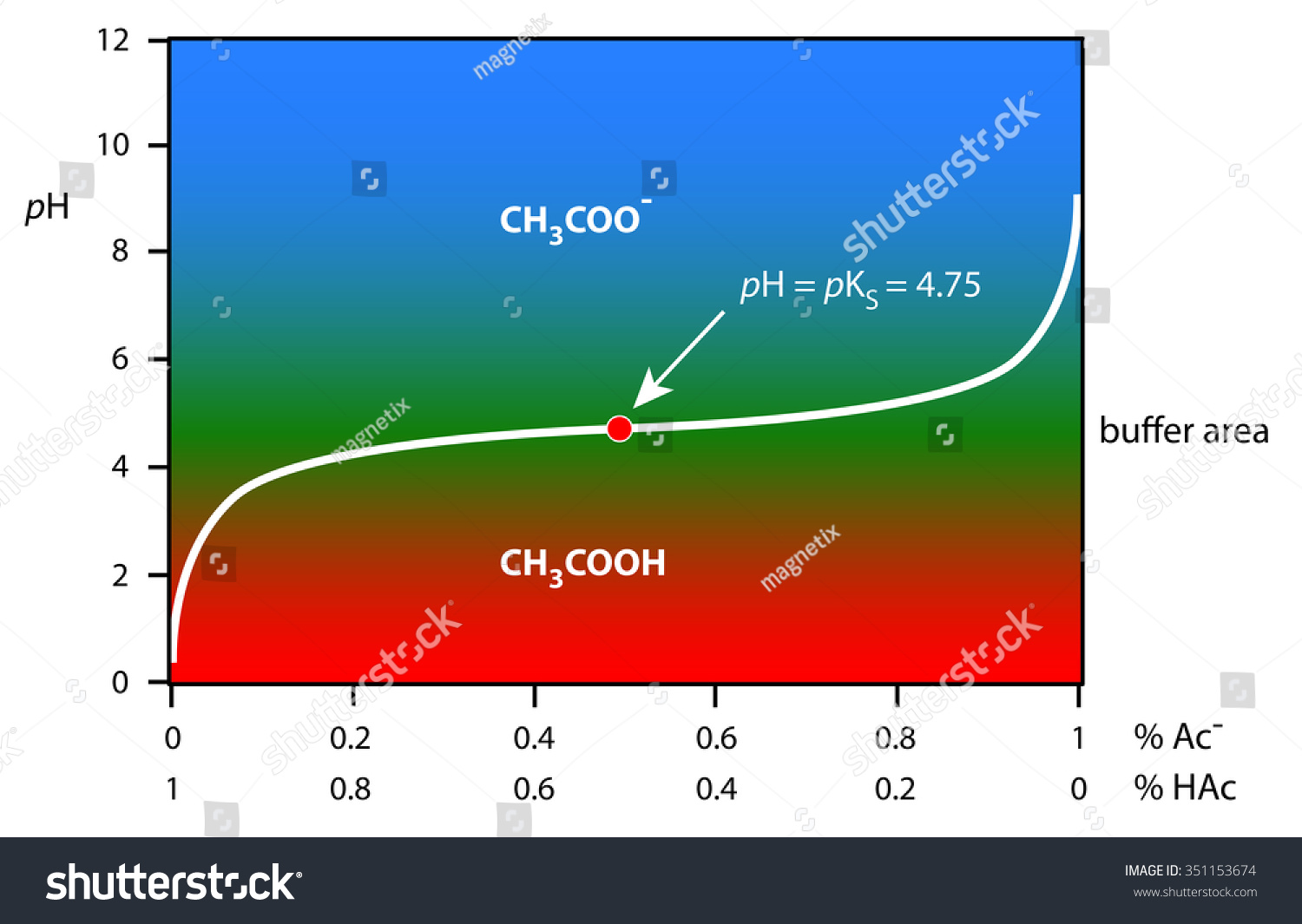
Buffer Curve Of Acetic Acid / Acetate System Stock Photo 351153674
Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. Consider the dissociation equation for acetic acid: A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. An example of a buffer that consists of a weak. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. It is a buffer because it contains both.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid Buffer Equation To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. An example of a buffer that consists of a weak. It is a buffer. Acetic Acid Buffer Equation.
From byjus.com
When a small amount of HCL is added to a buffer solution of acetic acid Acetic Acid Buffer Equation A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. A buffer system can. Acetic Acid Buffer Equation.
From www.youtube.com
What is the pH of buffer solution containing 0.17 M acetic acid and 0. Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are. Acetic Acid Buffer Equation.
From general.chemistrysteps.com
pH of a Buffer Solution Chemistry Steps Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. A mixture of acetic acid and sodium acetate is acidic because the. Acetic Acid Buffer Equation.
From www.numerade.com
SOLVED 4. The pH of a sodium acetateacetic acid buffer is 4.5 Acetic Acid Buffer Equation An example of a buffer that consists of a weak. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Consider the dissociation equation for acetic acid:. Acetic Acid Buffer Equation.
From www.slideserve.com
PPT Equilibrium Acids and Bases PowerPoint Presentation, free Acetic Acid Buffer Equation An example of a buffer that consists of a weak. Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. It is a buffer because it contains both. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. Buffer solutions resist. Acetic Acid Buffer Equation.
From www.slideserve.com
PPT Buffers and the HendersonHasselbalch Equation PowerPoint Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. It is a buffer because it contains both. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Hac ⇌ h + + ac¯ increasing the concentration of the. Acetic Acid Buffer Equation.
From www.shutterstock.com
Buffer Curve Of Acetic Acid / Acetate System Stock Photo 351153674 Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. An example of a buffer that consists of a weak. Consider the. Acetic Acid Buffer Equation.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID1149749 Acetic Acid Buffer Equation To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. Buffer solutions resist a change in ph when small amounts of a strong acid. Acetic Acid Buffer Equation.
From www.chegg.com
Solved 7. Write a balanced equation for the dissociation Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer. Acetic Acid Buffer Equation.
From www.numerade.com
SOLVED Write an equation showing how this buffer neutralizes added Acetic Acid Buffer Equation A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. Consider the dissociation equation for acetic acid: Buffer solutions resist a change in ph when small. Acetic Acid Buffer Equation.
From criticalthinking.cloud
how to solve buffer problems chemistry Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. Buffer solutions resist a change in ph when small amounts of. Acetic Acid Buffer Equation.
From www.numerade.com
SOLVED Use the HendersonHasselbalch equation to perform the following Acetic Acid Buffer Equation It is a buffer because it contains both. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Consider the dissociation. Acetic Acid Buffer Equation.
From www.numerade.com
SOLVED A) A buffer solution made from acetic acid (CH3COOH) and sodium Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. Consider the dissociation equation for acetic acid: An example of a. Acetic Acid Buffer Equation.
From www.chegg.com
Solved Complete each of the following chemical reactions for Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid. Acetic Acid Buffer Equation.
From byjus.com
For preparing a buffer of pH 6 by mixing sodium acetate and acetic acid Acetic Acid Buffer Equation To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. It is a buffer because it contains both. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. An example of a buffer that consists of a. Acetic Acid Buffer Equation.
From www.tessshebaylo.com
Equilibrium Equation For Ionization Of Acetic Acid Tessshebaylo Acetic Acid Buffer Equation It is a buffer because it contains both. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. To illustrate the function of a buffer solution,. Acetic Acid Buffer Equation.
From clarissa-bloggay.blogspot.com
Balanced Equation for the Neutralization of Acetic Acid Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. An example of a buffer that consists of a weak. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. To illustrate the function of a. Acetic Acid Buffer Equation.
From www.chegg.com
Solved Making 100 mL of acetate/acetic acid buffer at pH 5. Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are. Acetic Acid Buffer Equation.
From allyson-has-mccoy.blogspot.com
Which Pair Will Produce a Buffer Solution AllysonhasMccoy Acetic Acid Buffer Equation A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. It is a buffer because it contains both. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A solution of. Acetic Acid Buffer Equation.
From www.chegg.com
Solved A buffer contains significant amounts of acetic Acetic Acid Buffer Equation Consider the dissociation equation for acetic acid: A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid. Acetic Acid Buffer Equation.
From people.chem.umass.edu
Chem 112 Lecture Spring 01 Overheads Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. To illustrate the function of a buffer solution, consider a mixture of. Acetic Acid Buffer Equation.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. An example of a buffer that consists of a weak. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. To illustrate the function of a buffer solution, consider a mixture. Acetic Acid Buffer Equation.
From www.chegg.com
Solved 2. A buffer solution contains 0.120 M acetic acid and Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A solution of acetic acid and sodium acetate (ch 3 cooh + ch 3 coona) is an example of a buffer that consists of a weak acid and its salt. Consider the dissociation equation for acetic acid:. Acetic Acid Buffer Equation.
From courses.lumenlearning.com
Buffers Chemistry Atoms First Acetic Acid Buffer Equation To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. An example of a buffer that consists of a weak. Consider the dissociation equation. Acetic Acid Buffer Equation.
From www.coursehero.com
[Solved] 1.The pH of a sodium acetateacetic acid buffer is 4.50 Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. Buffer solutions resist a change in ph when small amounts of. Acetic Acid Buffer Equation.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Acetic Acid Buffer Equation Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. Consider the dissociation equation for acetic acid: A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. It is a buffer because it contains both. A. Acetic Acid Buffer Equation.
From www.numerade.com
SOLVED 'Write reaction equations to explain how your acetic acid Acetic Acid Buffer Equation To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate. Consider the dissociation equation for acetic acid: It is a buffer because it contains. Acetic Acid Buffer Equation.
From www.slideshare.net
Ch 18 buffers Acetic Acid Buffer Equation A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. An example of a buffer that consists of a weak. Buffer solutions resist a change in. Acetic Acid Buffer Equation.
From www.slideserve.com
PPT AcidBase Equilibria PowerPoint Presentation, free download ID Acetic Acid Buffer Equation A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. It is a buffer because it contains both. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Buffer solutions resist a change in. Acetic Acid Buffer Equation.
From general.chemistrysteps.com
The Common Ion Effect Chemistry Steps Acetic Acid Buffer Equation To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. It is a buffer because it contains both. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. A buffer system can be made. Acetic Acid Buffer Equation.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Acetic Acid Buffer Equation An example of a buffer that consists of a weak. Consider the dissociation equation for acetic acid: Hac ⇌ h + + ac¯ increasing the concentration of the acetate (ac¯) will push the equiibrium back. A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base. Acetic Acid Buffer Equation.
From www.coursehero.com
[Solved] Use the HendersonHasselbach equation to calculate the initial Acetic Acid Buffer Equation It is a buffer because it contains both. Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure \ (\pageindex. An example of a buffer that consists of a weak. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and. Acetic Acid Buffer Equation.
From www.tessshebaylo.com
Choose The Correct Chemical Equation For Ionization Of Acetic Acid Acetic Acid Buffer Equation Buffer solutions resist a change in ph when small amounts of a strong acid or a strong base are added (figure. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. An example of a buffer that consists of a weak. It is a buffer because it contains both. A. Acetic Acid Buffer Equation.
From www.myxxgirl.com
Acetic Acid And Sodium Acetate Equation My XXX Hot Girl Acetic Acid Buffer Equation Consider the dissociation equation for acetic acid: A mixture of acetic acid and sodium acetate is acidic because the ka of acetic acid is greater than the kb of its conjugate base acetate. To illustrate the function of a buffer solution, consider a mixture of roughly equal amounts of acetic acid and sodium. Buffer solutions resist a change in ph. Acetic Acid Buffer Equation.