Ring Formation In Organic Chemistry . Alkyl shifts adjacent to strained rings (e.g. In this fascinating example, that results. This reaction plays a crucial role in. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. Cyclobutane) can result in ring expansion. The ring closure reaction of a reactive intermediate could be one of the three types. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. The net effect is the shifting over of the carbocation to the adjacent carbon. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond.
from www.masterorganicchemistry.com
Alkyl shifts adjacent to strained rings (e.g. This reaction plays a crucial role in. The ring closure reaction of a reactive intermediate could be one of the three types. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. In this fascinating example, that results. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. The net effect is the shifting over of the carbocation to the adjacent carbon. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. Cyclobutane) can result in ring expansion. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation.
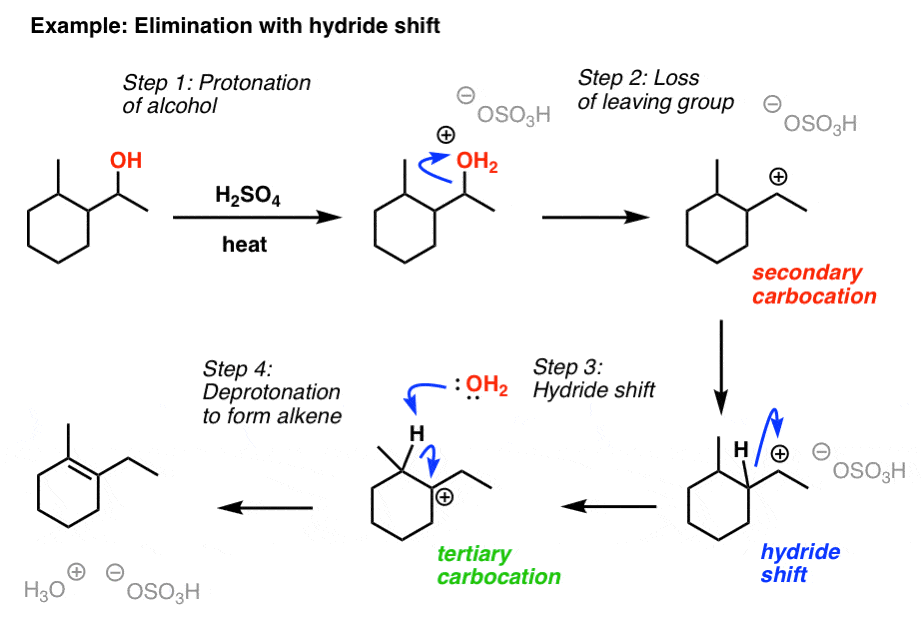
Elimination Reactions of Alcohols Master Organic Chemistry
Ring Formation In Organic Chemistry Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. The net effect is the shifting over of the carbocation to the adjacent carbon. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. Alkyl shifts adjacent to strained rings (e.g. This reaction plays a crucial role in. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. In this fascinating example, that results. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. The ring closure reaction of a reactive intermediate could be one of the three types. Cyclobutane) can result in ring expansion.
From www.pinterest.com
Organische Ringverbindungen, organische ringverbindungen Organic Ring Formation In Organic Chemistry The net effect is the shifting over of the carbocation to the adjacent carbon. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. In this fascinating example, that results. Cyclobutane) can result in ring expansion. Formation of the ketone promotes rearrangement of the ring bond to force out. Ring Formation In Organic Chemistry.
From pubs.acs.org
SevenMembered Ring Formation from Cyclopropanated Oxo and Ring Formation In Organic Chemistry The net effect is the shifting over of the carbocation to the adjacent carbon. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Alkyl shifts adjacent to strained rings (e.g. A cyclization reaction refers to the formation of a ring compound from a chain. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Epoxide Ring Opening With Base Master Organic Chemistry Ring Formation In Organic Chemistry The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. The net effect is the shifting over of the carbocation to the adjacent carbon. Cyclobutane) can result in ring expansion. This reaction. Ring Formation In Organic Chemistry.
From www.youtube.com
Organic chemistry Tricks for Ring Expansion and Ring Contraction YouTube Ring Formation In Organic Chemistry The net effect is the shifting over of the carbocation to the adjacent carbon. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. This reaction plays a crucial role in. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. A cyclization reaction. Ring Formation In Organic Chemistry.
From www.pinterest.jp
Five and SixMembered Rings are Favorable in Intramolecular Aldol Ring Formation In Organic Chemistry Alkyl shifts adjacent to strained rings (e.g. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. Cyclobutane) can result in ring expansion. In this fascinating example, that results. The net effect. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Summary Alkene Reaction Pathways — Master Organic Chemistry Ring Formation In Organic Chemistry The ring closure reaction of a reactive intermediate could be one of the three types. Cyclobutane) can result in ring expansion. The net effect is the shifting over of the carbocation to the adjacent carbon. In this fascinating example, that results. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one. Ring Formation In Organic Chemistry.
From openpress.usask.ca
9.5. Nomenclature Introduction to Organic Chemistry Ring Formation In Organic Chemistry This reaction plays a crucial role in. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. The net effect is the shifting over of the carbocation to the adjacent carbon. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory”. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
The E2 Reaction and Cyclohexane Rings Master Organic Chemistry Ring Formation In Organic Chemistry This reaction plays a crucial role in. The ring closure reaction of a reactive intermediate could be one of the three types. In this fascinating example, that results. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. The net effect is the shifting over of the carbocation to the adjacent carbon. Alkyl. Ring Formation In Organic Chemistry.
From www.pinterest.cl
ALKENES Organic chemistry, Organic chemistry reactions, Organic Ring Formation In Organic Chemistry This reaction plays a crucial role in. Alkyl shifts adjacent to strained rings (e.g. The ring closure reaction of a reactive intermediate could be one of the three types. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Cyclobutane) can result in ring expansion.. Ring Formation In Organic Chemistry.
From www.slideserve.com
PPT Reactions of a Hydrogens Condensation Reactions PowerPoint Ring Formation In Organic Chemistry Alkyl shifts adjacent to strained rings (e.g. This reaction plays a crucial role in. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Cyclobutane) can. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Reaction of epoxides with nucleophiles under basic conditions Master Ring Formation In Organic Chemistry The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. In this fascinating example, that results. Cyclobutane) can result in ring expansion. This reaction plays a crucial role in. The net effect is the shifting over of the carbocation to the adjacent carbon. Alkyl shifts are most likely to. Ring Formation In Organic Chemistry.
From www.pinterest.es
RING CONFORMATION Organic chemistry study, Study chemistry, Chemistry Ring Formation In Organic Chemistry Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. A cyclization reaction refers to the formation of a ring compound from a chain by the. Ring Formation In Organic Chemistry.
From www.slideserve.com
PPT 30. Orbitals and Organic Chemistry Pericyclic Reactions Ring Formation In Organic Chemistry Cyclobutane) can result in ring expansion. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. In this fascinating example, that results. This reaction plays a crucial role. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Imines Properties, Formation, Reactions, and Mechanisms Master Ring Formation In Organic Chemistry Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical. Ring Formation In Organic Chemistry.
From chemistry.com.pk
Functional Groups in Organic Chemistry [Infographic] Ring Formation In Organic Chemistry Cyclobutane) can result in ring expansion. The net effect is the shifting over of the carbocation to the adjacent carbon. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. In this fascinating example, that results. The ring closure reaction of a reactive intermediate could be one of the. Ring Formation In Organic Chemistry.
From www.youtube.com
Organic Chemistry II Ring Closing Metathesis YouTube Ring Formation In Organic Chemistry The ring closure reaction of a reactive intermediate could be one of the three types. Cyclobutane) can result in ring expansion. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to. Ring Formation In Organic Chemistry.
From www.neetexambooster.in
Organic chemistry Some basic principles and processes Important Ring Formation In Organic Chemistry This reaction plays a crucial role in. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal. Ring Formation In Organic Chemistry.
From www.youtube.com
Organic Chemistry Ring Expansion YouTube Ring Formation In Organic Chemistry This reaction plays a crucial role in. Cyclobutane) can result in ring expansion. The ring closure reaction of a reactive intermediate could be one of the three types. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a. Ring Formation In Organic Chemistry.
From www.chemistrysteps.com
Epoxides RingOpening Reactions Chemistry Steps Ring Formation In Organic Chemistry This reaction plays a crucial role in. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. Cyclobutane) can result in ring expansion. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. The ring closure reaction of a reactive. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Introduction to Cycloalkanes Two Key Consequences of Ring Formation Ring Formation In Organic Chemistry A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. This reaction plays a crucial role in. Formation of the ketone promotes rearrangement of. Ring Formation In Organic Chemistry.
From www.youtube.com
Test yourself solution to organic chemistry Tricks for Ring expansion Ring Formation In Organic Chemistry Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. Alkyl shifts adjacent to strained rings (e.g. The ring closure reaction of a reactive intermediate could be one of the. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Intramolecular Williamson Ether Synthesis Master Organic Chemistry Ring Formation In Organic Chemistry A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. In this fascinating example, that results. The ring closure reaction of a reactive intermediate could be one of the three. Ring Formation In Organic Chemistry.
From www.numerade.com
SOLVEDCarbon ring structures are common in organic chemistry. Draw a Ring Formation In Organic Chemistry A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. This reaction plays a crucial role in. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. The net effect is the shifting over of the carbocation to the adjacent carbon. In. Ring Formation In Organic Chemistry.
From www.youtube.com
Naming Compounds with Ring Branches (IUPAC Style) Organic Chemistry I Ring Formation In Organic Chemistry This reaction plays a crucial role in. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. The ring closure reaction of a reactive intermediate could be one of the three types. Cyclobutane) can result in ring expansion. In this fascinating example, that results. Alkyl shifts adjacent to strained rings (e.g. The net. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Summary Alkene Reaction Pathways — Master Organic Chemistry Ring Formation In Organic Chemistry Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. In this fascinating example, that results. The ring closure reaction of a reactive intermediate could be one of the three types. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. This reaction plays. Ring Formation In Organic Chemistry.
From www.chemtube3d.com
Intramolecular aldol reactions (Eightmembered ring formation) Ring Formation In Organic Chemistry Cyclobutane) can result in ring expansion. The ring closure reaction of a reactive intermediate could be one of the three types. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into. Ring Formation In Organic Chemistry.
From www.youtube.com
Ring Contraction organic chemistry Rearrangement of carbocations Ring Formation In Organic Chemistry Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. In this fascinating example, that results. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. Alkyl shifts adjacent to strained rings (e.g. The net effect is the shifting over. Ring Formation In Organic Chemistry.
From www.pinterest.com
Organic ring compounds, organic compounds Education subject Ring Formation In Organic Chemistry Cyclobutane) can result in ring expansion. This reaction plays a crucial role in. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. The ring closure. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Elimination Reactions of Alcohols Master Organic Chemistry Ring Formation In Organic Chemistry In this fascinating example, that results. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. The net effect is the shifting over of the carbocation to the adjacent carbon. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. This reaction plays a. Ring Formation In Organic Chemistry.
From www.masterorganicchemistry.com
Elimination Reactions of Alcohols Master Organic Chemistry Ring Formation In Organic Chemistry The ring closure reaction of a reactive intermediate could be one of the three types. Cyclobutane) can result in ring expansion. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure. Ring Formation In Organic Chemistry.
From www.doubtnut.com
Draw chain and ring structures of organic compound having six carbon a Ring Formation In Organic Chemistry Alkyl shifts adjacent to strained rings (e.g. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. This reaction plays a crucial role in. Cyclobutane) can result in ring expansion. Alkyl shifts. Ring Formation In Organic Chemistry.
From www.youtube.com
organic chemistry reaction mechanism RING EXPANSION Neeraj dubey Ring Formation In Organic Chemistry We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Alkyl shifts adjacent to strained rings (e.g. This reaction plays a crucial role in. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence.. Ring Formation In Organic Chemistry.
From api-project-1022638073839.appspot.com
How would you know if a cyclic organic molecule is planar? Socratic Ring Formation In Organic Chemistry A cyclization reaction refers to the formation of a ring compound from a chain by the creation of a new bond. Cyclobutane) can result in ring expansion. In this fascinating example, that results. We saw that systems with 4pi electrons undergo “conrotatory” ring closure (and opening) under thermal conditions, and “disrotatory” ring closure (and opening) under photochemical conditions. Alkyl shifts. Ring Formation In Organic Chemistry.
From www.studyorgo.com
Epoxide Reactions Organic Chemistry Help Ring Formation In Organic Chemistry This reaction plays a crucial role in. In this fascinating example, that results. Alkyl shifts adjacent to strained rings (e.g. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. Alkyl shifts are most likely to occur when a quaternary carbon is adjacent to a secondary (or primary) carbocation.. Ring Formation In Organic Chemistry.
From www.studypool.com
SOLUTION Igcse chemistry organic chemistry Studypool Ring Formation In Organic Chemistry The ring closure reaction of a reactive intermediate could be one of the three types. Formation of the ketone promotes rearrangement of the ring bond to force out the tosylate leaving group. In this fascinating example, that results. The robinson annulation is the name for a process that combines two key reactions you’ve learned previously into one longer sequence. Cyclobutane). Ring Formation In Organic Chemistry.