Torsional Strain Example . So, at any angle other than 60, 120, or. This organic chemistry video tutorial provides a basic introduction into torsional strain and. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Any deviation from staggered conformation causes torsional strain. The occurrence of torsional strain is explained via. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. And in order for this to be considered. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain.
from www.chegg.com
And in order for this to be considered. Any deviation from staggered conformation causes torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. The occurrence of torsional strain is explained via. This organic chemistry video tutorial provides a basic introduction into torsional strain and. So, at any angle other than 60, 120, or. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms.
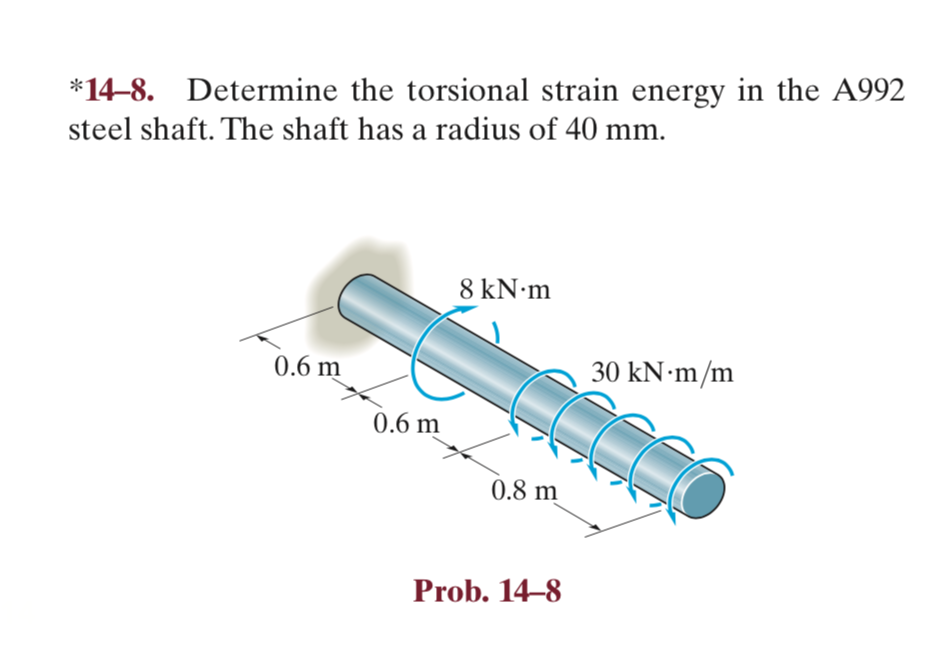
Solved *148. Determine the torsional strain energy in the
Torsional Strain Example Any deviation from staggered conformation causes torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. So, at any angle other than 60, 120, or. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Any deviation from staggered conformation causes torsional strain. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. The occurrence of torsional strain is explained via. And in order for this to be considered.
From www.youtube.com
Torsional Strain and Steric Strain YouTube Torsional Strain Example So, at any angle other than 60, 120, or. And in order for this to be considered. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. Torsional strain is the difference in energy between the staggered and eclipsed conformations. Torsional Strain Example.
From slidetodoc.com
Torsional Deformation of a circular shaft Torsion Formula Torsional Strain Example The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Any deviation from staggered conformation causes. Torsional Strain Example.
From www.youtube.com
Strain energy for axial, torsional ,shear and bending case [In Hindi Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. So, at any angle other than 60, 120, or. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Torsional. Torsional Strain Example.
From www.youtube.com
Shearing strain due to torsion in a shaft YouTube Torsional Strain Example And in order for this to be considered. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. Torsional strain or eclipsing strain is the increase. Torsional Strain Example.
From www.youtube.com
2nd civilLec 2 (Shear Stresses due to Torsion ,Torsion of Solid and Torsional Strain Example Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. Any deviation from staggered conformation causes torsional strain. The occurrence of torsional strain is explained via. So, at any angle other than 60, 120, or. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. The increase in energy. Torsional Strain Example.
From www.youtube.com
Torsional stress part2, Free body diagram and examples YouTube Torsional Strain Example So, at any angle other than 60, 120, or. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. The occurrence of torsional strain is explained via. For simplicity's sake, torsional strain is defined as as the strain experienced by the. Torsional Strain Example.
From www.youtube.com
Calculate elastic strain energy for a member in torsion YouTube Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Any deviation from staggered conformation causes torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not. Torsional Strain Example.
From slidetodoc.com
Torsional Deformation of a circular shaft Torsion Formula Torsional Strain Example For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. So, at any angle other than 60, 120, or. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to. Torsional Strain Example.
From www.slideserve.com
PPT Cycloalkanes and their Stereochemistry PowerPoint Presentation Torsional Strain Example This organic chemistry video tutorial provides a basic introduction into torsional strain and. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Any deviation from staggered conformation causes torsional strain. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. The occurrence of torsional. Torsional Strain Example.
From www.slideserve.com
PPT Torsion PowerPoint Presentation, free download ID5554860 Torsional Strain Example And in order for this to be considered. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. Any deviation from staggered conformation causes torsional strain. This organic chemistry video tutorial provides a basic introduction into torsional strain and.. Torsional Strain Example.
From www.slideshare.net
Lecture7 123.101 Torsional Strain Example The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. So, at any angle other than 60, 120, or. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. The occurrence of torsional strain is explained via. For simplicity's. Torsional Strain Example.
From www.researchgate.net
Diagram of the concept for torsional strain in a fixedfree hollow Torsional Strain Example The occurrence of torsional strain is explained via. And in order for this to be considered. Any deviation from staggered conformation causes torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. So, at any angle other than 60, 120, or. This. Torsional Strain Example.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID622339 Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. The occurrence of torsional strain is explained via. And in order for this to be considered. Any deviation from staggered conformation causes torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds. Torsional Strain Example.
From www.youtube.com
Introduction to the Torsion Formula Mechanics of Materials YouTube Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. The occurrence of torsional strain is explained via. And in order for this to be considered. Any deviation from. Torsional Strain Example.
From www.slideserve.com
PPT Torsion Shear Stress & Twist (3.13.5) PowerPoint Presentation Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. So, at any angle other than 60, 120, or. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule.. Torsional Strain Example.
From www.youtube.com
Strength of Materials (Part 12 Example using the General Torsion Torsional Strain Example This organic chemistry video tutorial provides a basic introduction into torsional strain and. And in order for this to be considered. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Any deviation from staggered conformation causes torsional strain. The increase in energy resulting from eclipsing groups on adjacent carbons. Torsional Strain Example.
From chemistryscore.com
Torsional Strain Learn Chemistry Online ChemistryScore Torsional Strain Example For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. So, at any angle other than 60, 120, or. And in order for this. Torsional Strain Example.
From slidetodoc.com
3 CHAPTER MECHANICS OF MATERIALS Torsion MECHANICS OF Torsional Strain Example Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. So, at any angle other than 60, 120, or. This organic chemistry video tutorial provides a basic introduction into torsional strain and. For simplicity's sake, torsional strain is defined as as the strain experienced. Torsional Strain Example.
From app.jove.com
Bending and Torsional Moments Concept Mechanical Engineering JoVe Torsional Strain Example The occurrence of torsional strain is explained via. So, at any angle other than 60, 120, or. Any deviation from staggered conformation causes torsional strain. And in order for this to be considered. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. For simplicity's sake, torsional strain is defined as as the strain experienced. Torsional Strain Example.
From www.youtube.com
Lect 9 Torsional Stress Examples 13 YouTube Torsional Strain Example And in order for this to be considered. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. Any deviation from staggered conformation causes torsional strain. This organic chemistry. Torsional Strain Example.
From www.slideshare.net
Lecture 13 torsion in solid and hollow shafts 1 Torsional Strain Example For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. The occurrence of torsional strain is explained via. Any deviation from. Torsional Strain Example.
From www.youtube.com
Shearing stress due to torsion in a solid circular shaft YouTube Torsional Strain Example The occurrence of torsional strain is explained via. And in order for this to be considered. Any deviation from staggered conformation causes torsional strain. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds. Torsional Strain Example.
From pediaa.com
Difference Between Steric and Torsional Strain Definition Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. This organic chemistry video tutorial provides a basic introduction into torsional strain and. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. The occurrence of torsional strain is explained via. Torsional strain is the difference in energy between the. Torsional Strain Example.
From www.youtube.com
lecture 5 Torsion stresses YouTube Torsional Strain Example The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. This organic chemistry video tutorial provides a basic introduction into torsional strain and. So,. Torsional Strain Example.
From www.youtube.com
Cyclohexane , Angle and Torsional Strain YouTube Torsional Strain Example The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. So, at any angle other than 60, 120, or. This organic chemistry video tutorial. Torsional Strain Example.
From depositphotos.com
Torsional Strain Eclipsed Conformation Stock Vector Image by ©samjore Torsional Strain Example The occurrence of torsional strain is explained via. And in order for this to be considered. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. This organic chemistry. Torsional Strain Example.
From openpress.usask.ca
3.4. Types of Strain in Molecules Introduction to Organic Chemistry Torsional Strain Example For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Any deviation from staggered conformation causes torsional strain. So, at any angle other than 60, 120, or. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. And in order for this to be. Torsional Strain Example.
From byjus.com
What is a torsional strain? Torsional Strain Example The occurrence of torsional strain is explained via. So, at any angle other than 60, 120, or. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. This organic chemistry video tutorial provides a basic introduction into torsional strain. Torsional Strain Example.
From www.slideshare.net
Lecture 13 torsion in solid and hollow shafts 1 Torsional Strain Example The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion. Torsional Strain Example.
From www.chemistrysteps.com
Ring Strain Chemistry Steps Torsional Strain Example Any deviation from staggered conformation causes torsional strain. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. So, at any angle other than 60, 120, or. Torsional strain or eclipsing strain is the increase in potential energy of a molecule due to repulsion between electrons in bonds that do not share an. Torsional strain. Torsional Strain Example.
From www.youtube.com
Hollow vs. Solid Rod TORSIONAL Shearing Stress in 2 Minutes! YouTube Torsional Strain Example This organic chemistry video tutorial provides a basic introduction into torsional strain and. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. The occurrence of torsional strain is explained via. And in order for. Torsional Strain Example.
From www.chegg.com
Solved *148. Determine the torsional strain energy in the Torsional Strain Example So, at any angle other than 60, 120, or. Any deviation from staggered conformation causes torsional strain. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. This organic chemistry video tutorial provides a. Torsional Strain Example.
From www.slideserve.com
PPT Torsion Shear Stress & Twist (3.13.5) PowerPoint Presentation Torsional Strain Example Torsional interactions are the result of repulsive forces between the bonding electrons of neighboring atoms. And in order for this to be considered. The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. This organic chemistry video tutorial provides a basic introduction into torsional strain and. So, at any angle other than 60, 120, or.. Torsional Strain Example.
From www.slideserve.com
PPT Torsion PowerPoint Presentation, free download ID6872810 Torsional Strain Example The occurrence of torsional strain is explained via. This organic chemistry video tutorial provides a basic introduction into torsional strain and. Any deviation from staggered conformation causes torsional strain. For simplicity's sake, torsional strain is defined as as the strain experienced by the bonds when conformations are not staggered. Torsional strain is the difference in energy between the staggered and. Torsional Strain Example.
From www.slideserve.com
PPT Torsional Shaft PowerPoint Presentation, free download ID5639789 Torsional Strain Example The increase in energy resulting from eclipsing groups on adjacent carbons is called torsional strain. Torsional strain is the difference in energy between the staggered and eclipsed conformations of a molecule. So, at any angle other than 60, 120, or. And in order for this to be considered. The occurrence of torsional strain is explained via. This organic chemistry video. Torsional Strain Example.