Conductivity Of Strong Electrolytes Lab Report . an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. highly ionized substances are strong electrolytes. The ions carry the electric charge through the solution thus creating an electric current. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. strong electrolytes produce large numbers of ions, which result in high conductivity values. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. Weak electrolytes have a low. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution.
from www.chegg.com
strong electrolytes produce large numbers of ions, which result in high conductivity values. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. highly ionized substances are strong electrolytes. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. Weak electrolytes have a low. The ions carry the electric charge through the solution thus creating an electric current. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified.
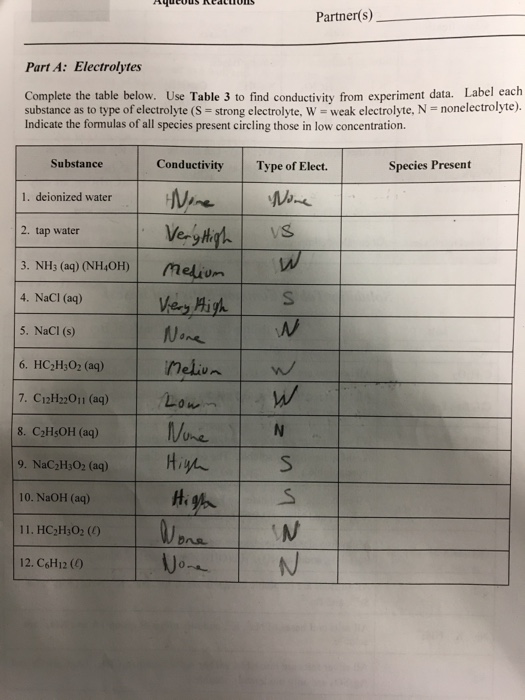
Solved Complete The Table Below. Use Table 3 To Find Cond...
Conductivity Of Strong Electrolytes Lab Report Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. strong electrolytes produce large numbers of ions, which result in high conductivity values. The ions carry the electric charge through the solution thus creating an electric current. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. highly ionized substances are strong electrolytes. Weak electrolytes have a low. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing.
From www.youtube.com
Electrolytes Conductivity Post Lab Discussion YouTube Conductivity Of Strong Electrolytes Lab Report The ions carry the electric charge through the solution thus creating an electric current. highly ionized substances are strong electrolytes. Weak electrolytes have a low. strong electrolytes produce large numbers of ions, which result in high conductivity values. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. . Conductivity Of Strong Electrolytes Lab Report.
From www.slideserve.com
PPT ELECTROLYTE CONDUCTANCE PowerPoint Presentation, free download Conductivity Of Strong Electrolytes Lab Report the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. Weak electrolytes have a low. The ions carry the electric charge through the solution thus creating an electric current. highly ionized substances are strong electrolytes. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate). Conductivity Of Strong Electrolytes Lab Report.
From studylib.net
Lab Conductivity of Solutions Conductivity Of Strong Electrolytes Lab Report The ions carry the electric charge through the solution thus creating an electric current. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. highly ionized substances are strong electrolytes. Weak electrolytes have a low. strong electrolytes produce large numbers of ions, which result in high conductivity values. . Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Weak electrolytes have a low. The ions carry the electric charge through the solution thus creating an. Conductivity Of Strong Electrolytes Lab Report.
From www.numerade.com
Experiment 6 Electrical Conductivity of Aqueous Solutions Electrolytes Conductivity Of Strong Electrolytes Lab Report in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. The ions carry the electric charge through the solution thus creating an electric current. strong electrolytes produce large numbers. Conductivity Of Strong Electrolytes Lab Report.
From www.researchgate.net
Composition and conductivity of electrolytes. Download Scientific Diagram Conductivity Of Strong Electrolytes Lab Report the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. strong electrolytes produce large numbers of ions,. Conductivity Of Strong Electrolytes Lab Report.
From www.thinkswap.com
Lab Report Discussing Conductivity of Strong and Weak Electrolytes in Conductivity Of Strong Electrolytes Lab Report Weak electrolytes have a low. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. an ionically conducting solution is called an. Conductivity Of Strong Electrolytes Lab Report.
From dokumen.tips
(PPTX) Solutions, Electrolytes, and Conductivity Lab 9. Purpose To Conductivity Of Strong Electrolytes Lab Report highly ionized substances are strong electrolytes. Weak electrolytes have a low. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. The ions carry the electric charge through the solution thus creating an electric current. an ionically conducting solution is called an electrolyte solution and the compound, which. Conductivity Of Strong Electrolytes Lab Report.
From dokumen.tips
(PDF) Conductivity of strong and weak electrolytes 06 · Conductivity of Conductivity Of Strong Electrolytes Lab Report Weak electrolytes have a low. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. The ions carry the electric charge through the solution thus creating an electric current. highly ionized substances are strong electrolytes. an ionically conducting solution is called an electrolyte solution and the compound, which produces. Conductivity Of Strong Electrolytes Lab Report.
From www.toppr.com
Statements(i) Unit of specific conductivity is ohm^{1} cm^{1}.(ii Conductivity Of Strong Electrolytes Lab Report highly ionized substances are strong electrolytes. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. Weak electrolytes have a low. The ions carry the electric charge through. Conductivity Of Strong Electrolytes Lab Report.
From chemistryprojectdickinson.weebly.com
Conductivity of Electrolytes Conductivity Of Strong Electrolytes Lab Report an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. Weak electrolytes have a low. highly ionized substances are strong electrolytes. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. strong electrolytes produce large numbers of ions, which result in high conductivity values.. Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. The ions carry the electric charge through the solution thus creating an electric current. in this lab. Conductivity Of Strong Electrolytes Lab Report.
From www.slideserve.com
PPT Post Lab Electrolytes PowerPoint Presentation, free download Conductivity Of Strong Electrolytes Lab Report in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. The ions carry the electric charge through the solution thus creating an electric current. measure the conductivity of the following. Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and. Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. in this lab you will explore the nature of aqueous solutions by investigating the relationship between. Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report strong electrolytes produce large numbers of ions, which result in high conductivity values. highly ionized substances are strong electrolytes. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Weak electrolytes have a low. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring. Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. The ions carry the electric charge through the solution thus creating an electric current. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. measure the conductivity of. Conductivity Of Strong Electrolytes Lab Report.
From cider.uoregon.edu
Conductivity Testing of Strong Electrolytes, Weak Electrolytes, and Non Conductivity Of Strong Electrolytes Lab Report strong electrolytes produce large numbers of ions, which result in high conductivity values. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. The ions carry. Conductivity Of Strong Electrolytes Lab Report.
From www.sarthaks.com
Explain the variation of molar conductivity with concentration for Conductivity Of Strong Electrolytes Lab Report measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. Weak electrolytes have a low. an ionically. Conductivity Of Strong Electrolytes Lab Report.
From www.slideserve.com
PPT Lab 11 Electrolytes and Conductivity PowerPoint Presentation Conductivity Of Strong Electrolytes Lab Report an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. strong electrolytes produce large numbers of ions, which result in high conductivity values. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. The ions carry the electric charge through the solution thus creating an. Conductivity Of Strong Electrolytes Lab Report.
From www.sarthaks.com
Explain with a graph the variation of molar conductivity of a strong Conductivity Of Strong Electrolytes Lab Report an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Weak electrolytes have a low. in this lab you will explore the nature of aqueous solutions by investigating the relationship between. Conductivity Of Strong Electrolytes Lab Report.
From www.chegg.com
Solved Complete The Table Below. Use Table 3 To Find Cond... Conductivity Of Strong Electrolytes Lab Report The ions carry the electric charge through the solution thus creating an electric current. strong electrolytes produce large numbers of ions, which result in high conductivity values. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. in this lab you will explore the nature of aqueous solutions by investigating the relationship. Conductivity Of Strong Electrolytes Lab Report.
From www.animalia-life.club
Electrolyte Strength Chart Conductivity Of Strong Electrolytes Lab Report Weak electrolytes have a low. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. highly ionized substances are strong electrolytes. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. measure the conductivity of the following solutions,. Conductivity Of Strong Electrolytes Lab Report.
From studylib.net
Experiment 4 Conductivity of electrolyte solutions Conductivity Of Strong Electrolytes Lab Report Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. Weak electrolytes have a low. in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. the ability of a substance to conduct electric current is due to the movement or migration of ions in. Conductivity Of Strong Electrolytes Lab Report.
From studylib.net
Conductivity of Electrolytes in Solution Conductivity Of Strong Electrolytes Lab Report the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. highly ionized substances are strong electrolytes. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Weak electrolytes have a low. The ions carry the electric charge. Conductivity Of Strong Electrolytes Lab Report.
From dokumen.tips
(PDF) Testing the Electrical Conductivity of Solid Electrolytes Conductivity Of Strong Electrolytes Lab Report the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. highly ionized substances are strong electrolytes. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Weak electrolytes have a low. in this lab you will explore. Conductivity Of Strong Electrolytes Lab Report.
From questions.kunduz.com
LABORATORY REPORT ELECTROLYTES A. Condu... Chemistry Conductivity Of Strong Electrolytes Lab Report measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. in this lab you will explore the nature of aqueous solutions by investigating the relationship between. Conductivity Of Strong Electrolytes Lab Report.
From www.researchgate.net
Conductivity data for various solid electrolyte materials Download Table Conductivity Of Strong Electrolytes Lab Report in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution.. Conductivity Of Strong Electrolytes Lab Report.
From www.studocu.com
Exp11 Conductometry new Experiment 11 2019 SAvzianova Conductometry Conductivity Of Strong Electrolytes Lab Report the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. . Conductivity Of Strong Electrolytes Lab Report.
From www.studypool.com
SOLUTION Lab7 electrical conductivity lab report Studypool Conductivity Of Strong Electrolytes Lab Report measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. highly ionized substances are strong electrolytes. strong electrolytes produce large numbers of ions, which result in high conductivity values. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as. Conductivity Of Strong Electrolytes Lab Report.
From www.vedantu.com
Lambda }^{\\infty }}of strong electrolytes can be obtained by Conductivity Of Strong Electrolytes Lab Report the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. Weak electrolytes have a low. the ability of a substance to conduct electric current is due to the movement or migration of ions in solution. strong electrolytes produce large numbers of ions, which result in high conductivity values.. Conductivity Of Strong Electrolytes Lab Report.
From www.chegg.com
Solved Report Sheet B. Electrolytes and Conductivity Conductivity Of Strong Electrolytes Lab Report in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. strong electrolytes produce large numbers of ions, which result in high conductivity values. The ions carry the electric charge through the solution thus creating an electric current. . Conductivity Of Strong Electrolytes Lab Report.
From www.scribd.com
Electrical Conductivity Lab Report Electrical Resistivity And Conductivity Of Strong Electrolytes Lab Report in this lab you will explore the nature of aqueous solutions by investigating the relationship between conductivity and. The ions carry the electric charge through the solution thus creating an electric current. the purpose of this lab is to differentiate between strong electrolytes, weak electrolytes, and nonelectrolytes by measuring and comparing. Weak electrolytes have a low. determine. Conductivity Of Strong Electrolytes Lab Report.
From dokumen.tips
(DOCX) MOLAR CONDUCTIVITY AT INFINITE DILUTION OF ELECTROLYTES LAB Conductivity Of Strong Electrolytes Lab Report Weak electrolytes have a low. an ionically conducting solution is called an electrolyte solution and the compound, which produces the ions as it dissolves, is called. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. determine whether samples are strong or weak electrolytes, nonelectrolytes, or unclassified. measure the conductivity of. Conductivity Of Strong Electrolytes Lab Report.
From www.studocu.com
Chem Lab 6 lab report Experiment 6 Electrolytes and Electrical Conductivity Of Strong Electrolytes Lab Report The ions carry the electric charge through the solution thus creating an electric current. highly ionized substances are strong electrolytes. measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Strong acids and salts are strong electrolytes because they completely ionize (dissociate or separate) in solution. strong. Conductivity Of Strong Electrolytes Lab Report.