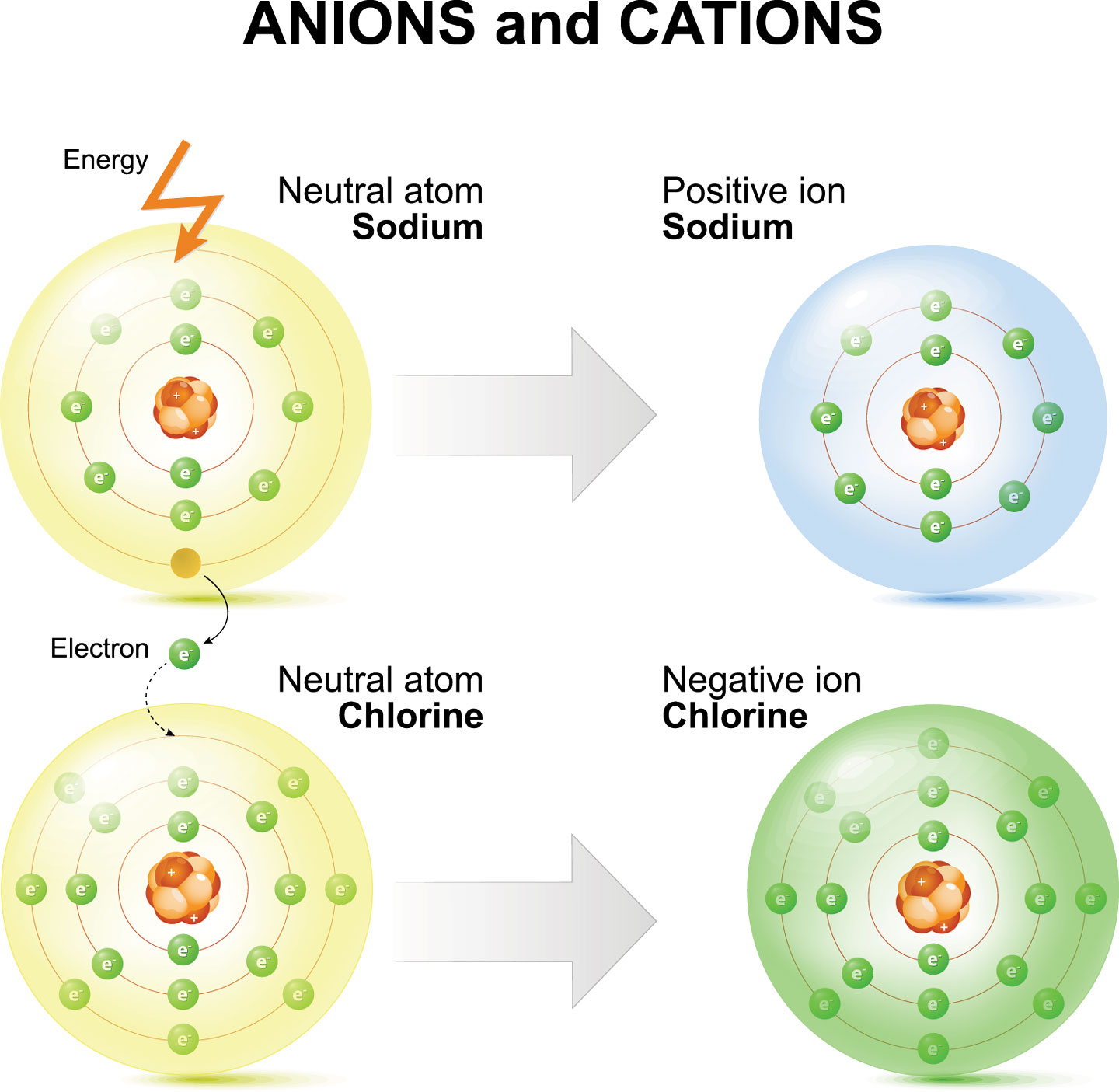
A Chlorine Atom Gains An Electron. What Is The Resulting Particle . What is the resulting particle? An isotope with no charge b. Most nonmetals become anions when they make ionic compounds. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. An isotope with a positive charge. What is the resulting particle? figure 3.1.2 3.1. Ions are formed by the transfer of electrons. What is formed when the chlorine. a chlorine atom gains an electron. a chlorine atom gains an electron. Example of ion charges and groups. An isotope with a positive. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram.
from www.snexplores.org
A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. An isotope with a positive. a chlorine atom gains an electron. What is the resulting particle? atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. An isotope with no charge b. Sulfur is in group 6 of the periodic. Example of ion charges and groups. An isotope with a positive charge. negatively charged ions are called anions.
Explainer Ions and radicals in our world
A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. What is the resulting particle? Most nonmetals become anions when they make ionic compounds. Ions are formed by the transfer of electrons. An isotope with no charge b. negatively charged ions are called anions. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. An isotope with a positive. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Sulfur is in group 6 of the periodic. a chlorine atom gains an electron. a chlorine atom gains an electron. An isotope with a positive charge. What is the resulting particle? Example of ion charges and groups. figure 3.1.2 3.1.
From www.expii.com
Ions — Definition & Overview Expii A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with a positive charge. figure 3.1.2 3.1. What is the resulting particle? What is formed when the chlorine. Most nonmetals become anions when they make ionic compounds. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. Example of ion charges and groups.. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of A Chlorine Atom Gains An Electron. What Is The Resulting Particle During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. Most nonmetals become anions when they make ionic compounds. An isotope with a positive charge. a chlorine atom gains an electron. figure 3.1.2 3.1. Ions are formed by the transfer of electrons. An isotope with no charge b. What is. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. Most nonmetals become anions when they make ionic compounds. Example of ion charges and groups. An isotope with no charge b. Ions are formed by the transfer of electrons. An isotope with a positive. a chlorine atom gains an electron. negatively charged ions are called anions. What is the resulting particle? A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica A Chlorine Atom Gains An Electron. What Is The Resulting Particle Example of ion charges and groups. negatively charged ions are called anions. Sulfur is in group 6 of the periodic. What is formed when the chlorine. An isotope with a positive charge. What is the resulting particle? Most nonmetals become anions when they make ionic compounds. What is the resulting particle? Ions are formed by the transfer of electrons. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From ar.inspiredpencil.com
Lewis Dot Structure Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle Ions are formed by the transfer of electrons. An isotope with a positive charge. What is the resulting particle? What is formed when the chlorine. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. atoms that lose electrons acquire a positive charge as a result because they are left with. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science A Chlorine Atom Gains An Electron. What Is The Resulting Particle During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. An isotope with a positive charge. a chlorine atom gains an electron. An isotope with no charge b. An isotope with a positive. negatively charged ions are called anions. a chlorine atom gains an electron. Most nonmetals become anions. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille A Chlorine Atom Gains An Electron. What Is The Resulting Particle Sulfur is in group 6 of the periodic. Example of ion charges and groups. Most nonmetals become anions when they make ionic compounds. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. a chlorine atom gains an electron. An isotope with a positive charge. An isotope with a positive. What is the resulting. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.youtube.com
Chlorine Electron Configuration YouTube A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. An isotope with no charge b. Ions are formed by the transfer of electrons. An isotope with a positive. negatively charged ions are called anions. Sulfur is in group 6 of the periodic. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. A. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From kunduz.com
[ANSWERED] The electron dot diagram for a neutral atom of chlorine Kunduz A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with no charge b. An isotope with a positive charge. What is the resulting particle? figure 3.1.2 3.1. Most nonmetals become anions when they make ionic compounds. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. a chlorine atom gains an electron. a chlorine atom gains. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From diagramtabormanages.z21.web.core.windows.net
Diagram Of Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle figure 3.1.2 3.1. a chlorine atom gains an electron. Most nonmetals become anions when they make ionic compounds. What is formed when the chlorine. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. Example of ion charges and groups. An isotope with a positive charge. What is the resulting. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From animalia-life.club
Lewis Dot Structure For Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle Ions are formed by the transfer of electrons. What is the resulting particle? What is formed when the chlorine. a chlorine atom gains an electron. What is the resulting particle? An isotope with a positive charge. Most nonmetals become anions when they make ionic compounds. An isotope with no charge b. An isotope with a positive. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with no charge b. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. What is formed when the chlorine. figure 3.1.2 3.1. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. An isotope with a positive.. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram A Chlorine Atom Gains An Electron. What Is The Resulting Particle What is the resulting particle? What is formed when the chlorine. An isotope with a positive. negatively charged ions are called anions. An isotope with no charge b. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. Most nonmetals become anions when they make ionic compounds. Sulfur is in group. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From exowfmlsz.blob.core.windows.net
Chlorine Ion Charge Formula at Angela Orlando blog A Chlorine Atom Gains An Electron. What Is The Resulting Particle A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. Most nonmetals become anions when they make ionic compounds. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. An isotope with a positive. An isotope with no charge b. What. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with a positive charge. a chlorine atom gains an electron. What is formed when the chlorine. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. figure 3.1.2 3.1. Example of ion charges and groups. An isotope with no charge b. What is the resulting particle? Most nonmetals. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) A Chlorine Atom Gains An Electron. What Is The Resulting Particle figure 3.1.2 3.1. An isotope with no charge b. What is formed when the chlorine. negatively charged ions are called anions. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. What is the resulting particle? Ions are formed by the transfer of electrons. Most nonmetals become anions when they. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle What is the resulting particle? Example of ion charges and groups. a chlorine atom gains an electron. What is the resulting particle? An isotope with no charge b. negatively charged ions are called anions. a chlorine atom gains an electron. figure 3.1.2 3.1. During a chemical reaction, a chlorine atom gains one electron from another atom. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science A Chlorine Atom Gains An Electron. What Is The Resulting Particle Ions are formed by the transfer of electrons. Most nonmetals become anions when they make ionic compounds. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. What is the resulting particle? negatively charged ions are called anions. A chlorine atom (cl) has equal numbers. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From gardenandplate.com
Molecules A Chlorine Atom Gains An Electron. What Is The Resulting Particle Ions are formed by the transfer of electrons. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. Sulfur is in group 6 of the periodic. figure 3.1.2 3.1. An isotope with a positive charge. Example of ion charges and groups. negatively charged ions are called anions. An isotope with no charge b.. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons A Chlorine Atom Gains An Electron. What Is The Resulting Particle A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. What is the resulting particle? atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. An isotope with no charge b. Most nonmetals become anions when they make ionic compounds. What. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. What is formed when the chlorine. Most nonmetals become anions when they make ionic compounds. negatively charged ions are called anions. figure 3.1.2 3.1. Ions are formed by the transfer of electrons. Sulfur is in group 6 of the periodic. Example of ion charges and groups. a chlorine atom gains. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model A Chlorine Atom Gains An Electron. What Is The Resulting Particle figure 3.1.2 3.1. negatively charged ions are called anions. What is the resulting particle? Most nonmetals become anions when they make ionic compounds. Ions are formed by the transfer of electrons. a chlorine atom gains an electron. An isotope with a positive. During a chemical reaction, a chlorine atom gains one electron from another atom as shown. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. figure 3.1.2 3.1. What is the resulting particle? During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. An isotope with a positive charge. What is formed when the chlorine. An isotope with a positive. atoms that lose electrons acquire a positive charge. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. figure 3.1.2 3.1. An isotope with a positive. Most nonmetals become anions when they make ionic compounds. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. a. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.numerade.com
SOLVED What change is occurring in this figure? Nat Sodium atom A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. negatively charged ions are called anions. Sulfur is in group 6 of the periodic. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.snexplores.org
Explainer Ions and radicals in our world A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. What is the resulting particle? atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. a chlorine atom gains an electron. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. Most nonmetals. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table A Chlorine Atom Gains An Electron. What Is The Resulting Particle What is formed when the chlorine. Most nonmetals become anions when they make ionic compounds. Example of ion charges and groups. What is the resulting particle? atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. An isotope with a positive. negatively charged ions are. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with no charge b. Ions are formed by the transfer of electrons. What is the resulting particle? Example of ion charges and groups. negatively charged ions are called anions. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. What is the resulting particle? a chlorine atom gains an electron. . A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From circuitetappesu.z4.web.core.windows.net
Atomic Diagram Of Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with a positive. negatively charged ions are called anions. What is the resulting particle? Sulfur is in group 6 of the periodic. figure 3.1.2 3.1. An isotope with no charge b. What is formed when the chlorine. An isotope with a positive charge. What is the resulting particle? A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains A Chlorine Atom Gains An Electron. What Is The Resulting Particle An isotope with a positive. figure 3.1.2 3.1. Sulfur is in group 6 of the periodic. a chlorine atom gains an electron. atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the. During a chemical reaction, a chlorine atom gains one electron from another. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library A Chlorine Atom Gains An Electron. What Is The Resulting Particle What is the resulting particle? What is the resulting particle? figure 3.1.2 3.1. Example of ion charges and groups. What is formed when the chlorine. Sulfur is in group 6 of the periodic. An isotope with a positive charge. Most nonmetals become anions when they make ionic compounds. During a chemical reaction, a chlorine atom gains one electron from. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From basichemistry.blogspot.com
Basic Chemistry October 2012 A Chlorine Atom Gains An Electron. What Is The Resulting Particle a chlorine atom gains an electron. During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. An isotope with no charge b. What is the resulting particle? negatively charged ions are called anions. Most nonmetals become anions when they make ionic compounds. a chlorine atom gains an electron. An. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements A Chlorine Atom Gains An Electron. What Is The Resulting Particle figure 3.1.2 3.1. Ions are formed by the transfer of electrons. Example of ion charges and groups. What is formed when the chlorine. An isotope with a positive charge. Sulfur is in group 6 of the periodic. What is the resulting particle? negatively charged ions are called anions. An isotope with no charge b. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From chemtech-us.com
15 Interesting Facts About Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle Ions are formed by the transfer of electrons. An isotope with a positive. negatively charged ions are called anions. What is the resulting particle? Example of ion charges and groups. An isotope with a positive charge. a chlorine atom gains an electron. A chlorine atom (cl) has equal numbers of protons and electrons (17) and is uncharged. Sulfur. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.
From guidelibefficience.z13.web.core.windows.net
Atomic Diagram Of Chlorine A Chlorine Atom Gains An Electron. What Is The Resulting Particle During a chemical reaction, a chlorine atom gains one electron from another atom as shown in the diagram. What is formed when the chlorine. Ions are formed by the transfer of electrons. negatively charged ions are called anions. What is the resulting particle? Example of ion charges and groups. What is the resulting particle? a chlorine atom gains. A Chlorine Atom Gains An Electron. What Is The Resulting Particle.