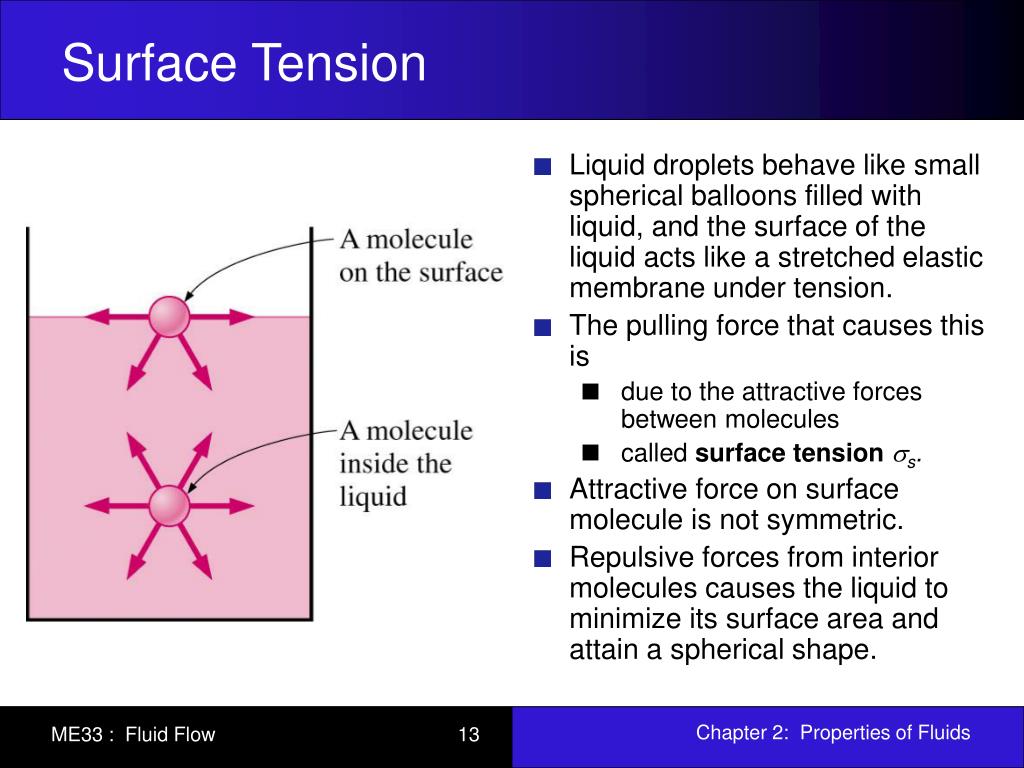
Surface Tension Liquid Zero . Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The floating water isn't in the shape of. But a molecule on the. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. This general effect is called surface tension. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The molecules at the surface. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero.
from www.slideserve.com
inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. The floating water isn't in the shape of. The molecules at the surface. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. But a molecule on the. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area.
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free
Surface Tension Liquid Zero Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. But a molecule on the. The floating water isn't in the shape of. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. The molecules at the surface. This general effect is called surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area.
From dxosoutiz.blob.core.windows.net
How To Calculate Water Surface Tension at Dana Zheng blog Surface Tension Liquid Zero But a molecule on the. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. The floating water isn't in the shape of. Molecules inside the liquid experience zero net. Surface Tension Liquid Zero.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Surface Tension Liquid Zero This general effect is called surface tension. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. But a molecule on the. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. if you look at the surface tension between water and water vapor as the critical point. Surface Tension Liquid Zero.
From www.researchgate.net
a) Surface tension of various common liquids in air are compared with Surface Tension Liquid Zero The molecules at the surface. The floating water isn't in the shape of. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. This general effect is called surface tension. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible. Surface Tension Liquid Zero.
From lessonschoolcarpino.z21.web.core.windows.net
How To Explain Surface Tension Surface Tension Liquid Zero This general effect is called surface tension. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension causes liquids to form spheres in free fall or zero gravity (see. Surface Tension Liquid Zero.
From www.youtube.com
Chemistry 8.2b Properties of Liquids Surface Tension and Capillary Surface Tension Liquid Zero This general effect is called surface tension. The molecules at the surface. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. surface tension causes liquids to form spheres in free. Surface Tension Liquid Zero.
From www.youtube.com
Measurement of surface tension Liquid state Bsc 1st year physical Surface Tension Liquid Zero The floating water isn't in the shape of. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. But a molecule on the. cohesive forces between molecules cause. Surface Tension Liquid Zero.
From exocmokjo.blob.core.windows.net
Liquid Have Surface Tension at Janice Curry blog Surface Tension Liquid Zero cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. But a molecule on the. if you look. Surface Tension Liquid Zero.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Liquid Zero if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. The floating water isn't in the shape of. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. the cohesive forces between. Surface Tension Liquid Zero.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical Surface Tension Liquid Zero Molecules inside the liquid experience zero net force, since they have neighbors on all sides. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. cohesive forces between molecules cause the surface of a liquid to contract. Surface Tension Liquid Zero.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid Surface Tension Liquid Zero This general effect is called surface tension. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. a molecule deep within the liquid is surrounded in all directions by other molecules, and so. Surface Tension Liquid Zero.
From byjus.com
80. A liquid does not wet the solid surface if the angle ofcontact is(b Surface Tension Liquid Zero cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. The floating water isn't in the shape of. This general effect is called surface tension. the cohesive forces between liquid molecules are responsible for the. Surface Tension Liquid Zero.
From www.youtube.com
Surface Tension [L4 Liquid Meniscus] NEET Physics l JEE Physicsl YouTube Surface Tension Liquid Zero Molecules inside the liquid experience zero net force, since they have neighbors on all sides. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Molecules on the. Surface Tension Liquid Zero.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension Liquid Zero The molecules at the surface. This general effect is called surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. The floating water isn't in the shape of. cohesive forces. Surface Tension Liquid Zero.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension Liquid Zero the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. cohesive forces between molecules cause the. Surface Tension Liquid Zero.
From www.youtube.com
Surface Tension Fluid Mechanics Fluid Science Liquid Droplet Surface Tension Liquid Zero surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. inshort surface tension occurs due to imbalance. Surface Tension Liquid Zero.
From pressbooks.online.ucf.edu
11.8 Cohesion and Adhesion in Liquids Surface Tension and Capillary Surface Tension Liquid Zero This general effect is called surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. inshort surface tension occurs due to imbalance of the intermolecular forces. Surface Tension Liquid Zero.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension Liquid Zero Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. The floating water isn't in the shape of.. Surface Tension Liquid Zero.
From www.youtube.com
Surface tension on liquid drop,soap bubble and liquid jet Surface Surface Tension Liquid Zero The molecules at the surface. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force. Surface Tension Liquid Zero.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Surface Tension Liquid Zero The molecules at the surface. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. This general effect is called surface tension. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The floating water isn't in the shape of. Molecules. Surface Tension Liquid Zero.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Surface Tension Liquid Zero The floating water isn't in the shape of. The molecules at the surface. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes.. Surface Tension Liquid Zero.
From dxozbnpql.blob.core.windows.net
Surface Tension In Water Definition at William Griffin blog Surface Tension Liquid Zero But a molecule on the. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. cohesive. Surface Tension Liquid Zero.
From circuitwiringtray.z13.web.core.windows.net
Surface Tension Of Water Diagram Surface Tension Liquid Zero the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. inshort. Surface Tension Liquid Zero.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension Liquid Zero a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. inshort surface tension occurs due to imbalance of the intermolecular forces of. Surface Tension Liquid Zero.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free Surface Tension Liquid Zero surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. The floating water. Surface Tension Liquid Zero.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension Liquid Zero if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. But a molecule on the. This general effect is called surface tension. The floating water isn't in the shape of. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. inshort. Surface Tension Liquid Zero.
From www.youtube.com
Lecture 3 Liquid state Applications of surface tension in daily life Surface Tension Liquid Zero The floating water isn't in the shape of. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. The molecules at the surface. Molecules inside the liquid experience zero net force,. Surface Tension Liquid Zero.
From www.diplomageeks.com
Define capilirity, viscosity, coefficient of viscosity and surface Surface Tension Liquid Zero The floating water isn't in the shape of. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. But a molecule on the. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface. Surface Tension Liquid Zero.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension Liquid Zero if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. But a molecule on the. a molecule deep within the liquid is surrounded in all directions by other molecules,. Surface Tension Liquid Zero.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru Surface Tension Liquid Zero a molecule deep within the liquid is surrounded in all directions by other molecules, and so the net force on it averages zero. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. Molecules inside the liquid experience zero net force, since they have neighbors on. Surface Tension Liquid Zero.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension Liquid Zero cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. But a molecule on the. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. The floating water isn't in. Surface Tension Liquid Zero.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Surface Tension Liquid Zero inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. Molecules. Surface Tension Liquid Zero.
From www.youtube.com
Surface Tension of Water Explained YouTube Surface Tension Liquid Zero the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. if you look at the surface tension between water and water vapor as the critical point is approached, the surface tension vanishes. This general effect is called surface tension. The floating water isn't in the shape of. Molecules on the surface are pulled. Surface Tension Liquid Zero.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs Surface Tension Liquid Zero Molecules inside the liquid experience zero net force, since they have neighbors on all sides. surface tension causes liquids to form spheres in free fall or zero gravity (see figure 2.4.1 2.4. the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. But a molecule on the. inshort surface tension occurs due. Surface Tension Liquid Zero.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension Liquid Zero The molecules at the surface. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. The floating water isn't in the shape of. This general effect is called surface tension. But a molecule on the. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. cohesive forces. Surface Tension Liquid Zero.
From www.youtube.com
What is Surface Tension Liquid State Liquid Dynamics Surface Surface Tension Liquid Zero the cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. Molecules inside the liquid experience zero net force, since they have neighbors on all sides. inshort surface tension occurs due to imbalance of the intermolecular forces of attraction on water molecules. if you look at the surface tension between water and water. Surface Tension Liquid Zero.