Does Saline Solution Freeze . The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. No need for complex instructions or additional steps—just a simple saline soak for your lenses. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. By keeping your saline solution in. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. With saline solution, contact lens cleaning and storage become a breeze. As saline solution moves through your nasal passages, it: Moistens nasal passages exposed to dry indoor air. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. The freezing point of saltwater is.
from www.gosupps.com
By keeping your saline solution in. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. The freezing point of saltwater is. As saline solution moves through your nasal passages, it: No need for complex instructions or additional steps—just a simple saline soak for your lenses. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. With saline solution, contact lens cleaning and storage become a breeze. Moistens nasal passages exposed to dry indoor air. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it.
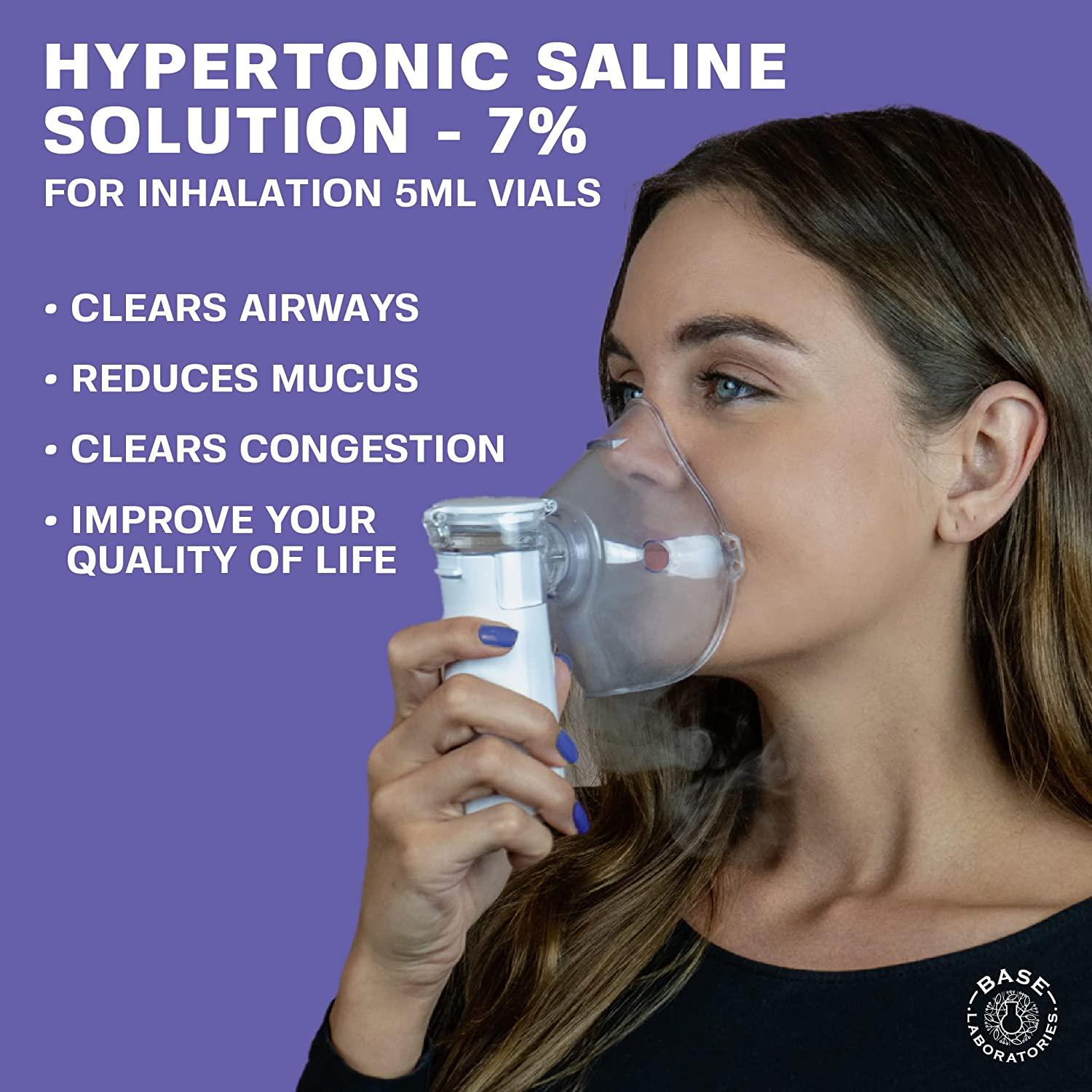
Base Labs 7 Hypertonic Saline Solution for Nebulizer Machine Sterile
Does Saline Solution Freeze No need for complex instructions or additional steps—just a simple saline soak for your lenses. The freezing point of saltwater is. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. With saline solution, contact lens cleaning and storage become a breeze. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. By keeping your saline solution in. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. As saline solution moves through your nasal passages, it: Moistens nasal passages exposed to dry indoor air. No need for complex instructions or additional steps—just a simple saline soak for your lenses.
From hoodootattoo.studio
Saline Solution How does it work? (Piercing Aftercare). Does Saline Solution Freeze If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. The freezing point of saltwater is. By keeping your saline solution in. Moistens nasal passages exposed to dry indoor air. The freezing point of a solution is always less than the freezing point of the pure solvent due to. Does Saline Solution Freeze.
From www.youtube.com
Saline Solution YouTube Does Saline Solution Freeze By keeping your saline solution in. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. As saline solution moves through your nasal passages, it: If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. No need for complex instructions or additional steps—just. Does Saline Solution Freeze.
From www.watercures.org
Saline IV Solution vs Water Cures Protocol The Salt Controversy? Does Saline Solution Freeze With saline solution, contact lens cleaning and storage become a breeze. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. Moistens nasal passages exposed to dry indoor air. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way. Does Saline Solution Freeze.
From www.warbyparker.com
What Is Saline Solution? Warby Parker Does Saline Solution Freeze Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. By keeping your saline solution in. No need for complex instructions or additional steps—just a simple saline soak for your lenses. With saline solution, contact lens cleaning and storage become a breeze. If you plan to use your saline solution within 24 hours. Does Saline Solution Freeze.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation Does Saline Solution Freeze Moistens nasal passages exposed to dry indoor air. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. With saline solution, contact lens cleaning and storage become a breeze. The freezing point of saltwater is. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. The freezing point. Does Saline Solution Freeze.
From www.metoffice.gov.uk
Sea ice an overview Met Office Does Saline Solution Freeze With saline solution, contact lens cleaning and storage become a breeze. The freezing point of saltwater is. No need for complex instructions or additional steps—just a simple saline soak for your lenses. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. Basically, the salt concentration in water (salinity) is measured as grams salt. Does Saline Solution Freeze.
From www.youtube.com
Saline Solution How to Make a Saline Solution YouTube Does Saline Solution Freeze With saline solution, contact lens cleaning and storage become a breeze. By keeping your saline solution in. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. As saline solution moves through your nasal passages, it: No need for complex instructions or additional steps—just a simple saline soak for. Does Saline Solution Freeze.
From www.alamy.com
Saline solution salt liquid water Stock Vector Images Alamy Does Saline Solution Freeze Moistens nasal passages exposed to dry indoor air. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. No need for complex instructions or additional steps—just a simple saline soak for your lenses. With saline solution, contact lens cleaning and storage become a breeze. Basically, the salt. Does Saline Solution Freeze.
From materialmcgheepearter.z21.web.core.windows.net
How Does Salt Affect Ice Does Saline Solution Freeze The freezing point of saltwater is. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. By keeping your saline solution in. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. The freezing point of a solution is always less than the. Does Saline Solution Freeze.
From www.wikidoc.org
Saline (medicine) wikidoc Does Saline Solution Freeze If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. Moistens nasal passages exposed to dry indoor air. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. No need for complex instructions or additional steps—just a simple saline soak for your. Does Saline Solution Freeze.
From www.amazon.com
Base Labs 7 Hypertonic Saline Solution for Nebulizer Does Saline Solution Freeze Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. As saline solution moves through your nasal. Does Saline Solution Freeze.
From www.nutriinspector.com
Can Salt Water Freeze Exploring the Salt's Frozen Depths Nutri Inspector Does Saline Solution Freeze As saline solution moves through your nasal passages, it: Moistens nasal passages exposed to dry indoor air. With saline solution, contact lens cleaning and storage become a breeze. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. Freezing saline solution can cause the solution to expand, potentially damaging. Does Saline Solution Freeze.
From www.slideserve.com
PPT What are Intravenous fluids ? PowerPoint Presentation, free Does Saline Solution Freeze Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. Moistens nasal passages exposed to dry indoor air. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to. Does Saline Solution Freeze.
From www.gosupps.com
Base Labs 7 Hypertonic Saline Solution for Nebulizer Machine Sterile Does Saline Solution Freeze With saline solution, contact lens cleaning and storage become a breeze. No need for complex instructions or additional steps—just a simple saline soak for your lenses. By keeping your saline solution in. As saline solution moves through your nasal passages, it: If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to. Does Saline Solution Freeze.
From jacksofscience.com
What Temp Does Salt Water Freeze? Jacks Of Science Does Saline Solution Freeze When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. The freezing point of saltwater is. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. With saline solution, contact lens cleaning and storage become a breeze. By keeping your saline. Does Saline Solution Freeze.
From www.slideserve.com
PPT Fluid and Electrolyte Therapy in the Pediatric Patient PowerPoint Does Saline Solution Freeze The freezing point of saltwater is. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. Moistens nasal passages exposed to dry indoor air. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. Basically, the salt concentration in water (salinity). Does Saline Solution Freeze.
From www.researchgate.net
Freezing point of salt solutions of different compositions; Cps is the Does Saline Solution Freeze The freezing point of saltwater is. With saline solution, contact lens cleaning and storage become a breeze. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. The freezing point of a. Does Saline Solution Freeze.
From edu.svet.gob.gt
IV Fluids (Intravenous Fluids) The Most Common Types Does Saline Solution Freeze By keeping your saline solution in. Moistens nasal passages exposed to dry indoor air. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. With saline solution, contact lens cleaning and storage become a breeze. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and. Does Saline Solution Freeze.
From www.biologyonline.com
Saline solution Definition and Examples Biology Online Dictionary Does Saline Solution Freeze Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. No need for complex instructions or additional steps—just a simple saline soak for your lenses. Freezing saline solution can cause the. Does Saline Solution Freeze.
From www.vrogue.co
Freezing Point Of Water Compared To A Salt Solution S vrogue.co Does Saline Solution Freeze Moistens nasal passages exposed to dry indoor air. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. The freezing point of saltwater is. As saline solution moves through your nasal passages, it: Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water.. Does Saline Solution Freeze.
From ldpwatersheds.org
How Does Salt Melt Snow and Ice? LDP Watersheds Does Saline Solution Freeze When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. By keeping your saline solution in. No need for complex instructions or additional steps—just a simple saline soak for your lenses. With saline solution, contact lens cleaning and storage become a breeze. The freezing point of saltwater is. The freezing point of a solution. Does Saline Solution Freeze.
From www.pinterest.com
Saltwater Experiment freeze water at different temperatures to see Does Saline Solution Freeze With saline solution, contact lens cleaning and storage become a breeze. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. Basically, the salt concentration in water (salinity) is measured. Does Saline Solution Freeze.
From www.pinterest.com
Saline solution refers to a salt solution, which you can prepare Does Saline Solution Freeze If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. By keeping your saline solution in. No need for complex instructions or additional steps—just a simple saline soak for your lenses. With saline solution, contact lens cleaning and storage become a breeze. As saline solution moves through your nasal. Does Saline Solution Freeze.
From karmahousecairns.com.au
A Guide To Best Body Piercing Aftercare Products Worldwide Tattoo Does Saline Solution Freeze As saline solution moves through your nasal passages, it: With saline solution, contact lens cleaning and storage become a breeze. No need for complex instructions or additional steps—just a simple saline soak for your lenses. The freezing point of saltwater is. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. When the. Does Saline Solution Freeze.
From www.gosupps.com
Base Labs 7 Hypertonic Saline Solution for Nebulizer Machine 25 Does Saline Solution Freeze The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. By keeping your saline solution in. The freezing point of saltwater is. Freezing saline solution can cause the solution to expand,. Does Saline Solution Freeze.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation Does Saline Solution Freeze The freezing point of saltwater is. With saline solution, contact lens cleaning and storage become a breeze. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. Basically, the salt concentration in. Does Saline Solution Freeze.
From www.gosupps.com
Base Labs 7 Hypertonic Saline Solution for Nebulizer Machine Sterile Does Saline Solution Freeze By keeping your saline solution in. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. The freezing point of saltwater is. With saline solution, contact lens cleaning and storage become a breeze. If you plan to use your saline solution within 24 hours of opening, refrigeration. Does Saline Solution Freeze.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation Does Saline Solution Freeze Moistens nasal passages exposed to dry indoor air. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. No need for complex instructions or additional steps—just a simple saline soak for your lenses. By keeping your saline solution in. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of. Does Saline Solution Freeze.
From www.youtube.com
physiological salt solution types, composition and uses YouTube Does Saline Solution Freeze Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. No need for complex instructions or additional steps—just a simple saline soak for your lenses. Moistens nasal passages exposed to dry indoor air. If you plan to use your saline solution within 24 hours of opening, refrigeration is an effective way to store it.. Does Saline Solution Freeze.
From www.vrogue.co
Freezing Point Of Water Compared To A Salt Solution S vrogue.co Does Saline Solution Freeze Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. The freezing point of saltwater is. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. By keeping your saline solution in. The freezing point of a solution is always less than the freezing point of the. Does Saline Solution Freeze.
From www.youtube.com
Saline solution recipe YouTube Does Saline Solution Freeze By keeping your saline solution in. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. Moistens nasal passages exposed to dry indoor air. Basically, the salt concentration in water (salinity) is measured as grams salt per kilogram of water. With saline solution, contact lens cleaning and. Does Saline Solution Freeze.
From nurse.plus
Common Hospital IV Drips Names, Types, and Their Uses Does Saline Solution Freeze By keeping your saline solution in. With saline solution, contact lens cleaning and storage become a breeze. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. Basically, the salt concentration. Does Saline Solution Freeze.
From www.flickr.com
Does Salt Water Freeze Pictures for Emily's Science Fair p… Flickr Does Saline Solution Freeze No need for complex instructions or additional steps—just a simple saline soak for your lenses. Moistens nasal passages exposed to dry indoor air. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. As saline solution moves through your nasal passages, it: If you plan to use your saline solution within 24 hours of. Does Saline Solution Freeze.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation Does Saline Solution Freeze Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. When the salinity is 15g/kg, its freezing point is 0.8°c, which goes on increasing for every. The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. With saline solution, contact. Does Saline Solution Freeze.
From www.medicalnewstoday.com
How to make saline solution at home Ingredients and uses Does Saline Solution Freeze The freezing point of a solution is always less than the freezing point of the pure solvent due to disruption of intermolecular interactions. By keeping your saline solution in. With saline solution, contact lens cleaning and storage become a breeze. Freezing saline solution can cause the solution to expand, potentially damaging the packaging and compromising its sterility. Basically, the salt. Does Saline Solution Freeze.