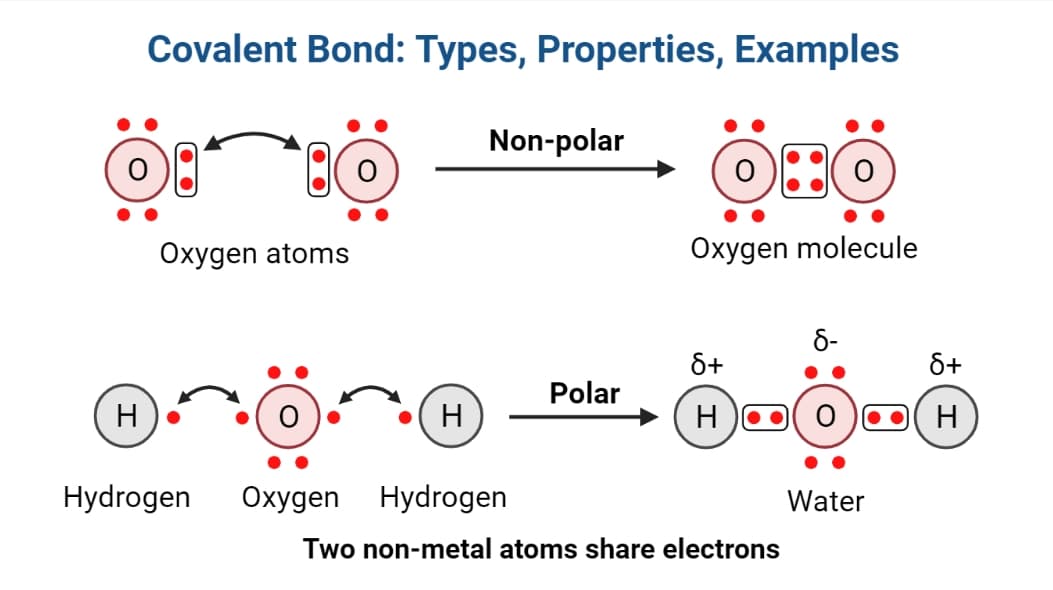
What Are The Three Types Of Chemical Bonds And How Are They Formed . types of chemical bonds not all chemical bonds form in the same way as the bonds in water. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. A bond between two atoms depends upon. There are actually three different types of. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. chemical bonds are described as having different strengths: the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. all models of chemical bonding have three common features: chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. Atoms form bonds because the products are more. A covalent bond is formed when atoms share valence electrons.
from www.phdnest.com
chemical bonds are described as having different strengths: A bond between two atoms depends upon. There are actually three different types of. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. all models of chemical bonding have three common features: A covalent bond is formed when atoms share valence electrons. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. Atoms form bonds because the products are more. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2].
Covalent Bond Meaning, Types, Properties, Examples PhD Nest
What Are The Three Types Of Chemical Bonds And How Are They Formed A covalent bond is formed when atoms share valence electrons. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. There are actually three different types of. A covalent bond is formed when atoms share valence electrons. Atoms form bonds because the products are more. chemical bonds are described as having different strengths: A bond between two atoms depends upon. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. all models of chemical bonding have three common features:
From learningschooldsbbbb56.z4.web.core.windows.net
Describe The Three Types Of Chemical Bonds What Are The Three Types Of Chemical Bonds And How Are They Formed the two main types of bonds formed between atoms are ionic bonds and covalent bonds. Atoms form bonds because the products are more. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. A bond between two atoms depends upon. the main types of. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From learningschooldsbbbb56.z4.web.core.windows.net
Describe The Three Types Of Chemical Bonds What Are The Three Types Of Chemical Bonds And How Are They Formed A bond between two atoms depends upon. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. A covalent bond is formed when atoms share valence electrons. the two main types. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideshare.net
Lesson 1 Intro to Chemical Bonding What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. chemical bonds are described as having different strengths: the two main types of bonds formed. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From tydesnhsteele.blogspot.com
List and Explain the Five Different Types of Bonds What Are The Three Types Of Chemical Bonds And How Are They Formed the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. types of chemical bonds not all chemical bonds form in the same way as the bonds in. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT CHEMICAL BONDS PowerPoint Presentation, free download ID6445592 What Are The Three Types Of Chemical Bonds And How Are They Formed There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. the two main. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. There are actually three different types of. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. Atoms form bonds because the products are more. There are strong bonds or primary. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From sciencenotes.org
Types of Chemical Bonds What Are The Three Types Of Chemical Bonds And How Are They Formed There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. A covalent bond is formed when atoms share valence electrons. types of chemical bonds not all chemical bonds form in the same way as the bonds in. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT TYPES OF CHEMICAL BONDS PowerPoint Presentation, free download What Are The Three Types Of Chemical Bonds And How Are They Formed the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. There are actually three different types of. An ionic bond is formed when one atom accepts or donates one or more of its. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.chemistrylearner.com
Covalent Bond Definition, Types, and Examples What Are The Three Types Of Chemical Bonds And How Are They Formed Atoms form bonds because the products are more. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. all models of chemical bonding have three common features: the two main. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Types of Primary Chemical Bonds PowerPoint Presentation, free What Are The Three Types Of Chemical Bonds And How Are They Formed the two main types of bonds formed between atoms are ionic bonds and covalent bonds. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. all models of chemical bonding have three common features: An ionic bond is formed when one atom accepts or. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.etsy.com
Types of Chemical Bonds Complete Set Types of Chemical Bonds Printable What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. A bond between two atoms depends upon. Atoms form bonds because the products are more. all models of chemical bonding have three common features: There are strong bonds or primary bonds such as covalent, ionic. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From learningschoolgroenselex.z4.web.core.windows.net
Types Of Chemical Bonds Explained What Are The Three Types Of Chemical Bonds And How Are They Formed An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. A covalent bond is formed when atoms share valence electrons. There are actually three different types of. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. the. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From biologydictionary.net
Covalent Bond Biology Dictionary What Are The Three Types Of Chemical Bonds And How Are They Formed the two main types of bonds formed between atoms are ionic bonds and covalent bonds. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. Atoms form bonds because the products are more. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Unit 14 Chemical Bonding PowerPoint Presentation, free download What Are The Three Types Of Chemical Bonds And How Are They Formed Atoms form bonds because the products are more. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. A bond between two atoms depends upon. the two main types of bonds. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Noble Gases and Valence e Ionization Energy and Bonding What Are The Three Types Of Chemical Bonds And How Are They Formed A bond between two atoms depends upon. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. all models of chemical bonding have three common features: the. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From learningschooldsbbbb56.z4.web.core.windows.net
Kinds Of Chemical Bonds What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds are described as having different strengths: There are actually three different types of. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. the two main types of bonds formed between atoms are. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica What Are The Three Types Of Chemical Bonds And How Are They Formed the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds are described as having different strengths: chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. types of chemical bonds not all chemical bonds form in the same. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.bartleby.com
Types of Chemical Bonds bartleby What Are The Three Types Of Chemical Bonds And How Are They Formed Atoms form bonds because the products are more. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. all models of chemical bonding have three common features: chemical bonds are described as having different strengths: chemical bonding, any of the interactions that account for the association of atoms. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.shutterstock.com
Chemical Bonds Infographic Diagram Showing Types Stock Illustration What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. A bond between two atoms depends upon. chemical bonds are described as having different strengths: chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood.. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Chapter 9 Chemical Bonding I Lewis Theory PowerPoint What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds are described as having different strengths: A bond between two atoms depends upon. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. the main types of chemical bonds are ionic bond,. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.phdnest.com
Covalent Bond Meaning, Types, Properties, Examples PhD Nest What Are The Three Types Of Chemical Bonds And How Are They Formed the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple,. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From materialmediakey77.z19.web.core.windows.net
Types Of Chemical Bonds Explained What Are The Three Types Of Chemical Bonds And How Are They Formed There are actually three different types of. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. A covalent bond is formed when atoms share valence electrons. There are strong. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Mastering Chemistry PowerPoint Presentation, free download ID What Are The Three Types Of Chemical Bonds And How Are They Formed types of chemical bonds not all chemical bonds form in the same way as the bonds in water. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds are described as having different strengths: There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,.. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Types of chemical bonds PowerPoint Presentation, free download What Are The Three Types Of Chemical Bonds And How Are They Formed types of chemical bonds not all chemical bonds form in the same way as the bonds in water. A covalent bond is formed when atoms share valence electrons. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when electrons can be simultaneously close to two or. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.biorender.com
Types of Chemical Bonds BioRender Science Templates What Are The Three Types Of Chemical Bonds And How Are They Formed There are actually three different types of. chemical bonds are described as having different strengths: Atoms form bonds because the products are more. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. A covalent bond is formed when atoms share valence electrons. There are strong bonds or primary bonds. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From xjfuxnexry.blogspot.com
Three Types Of Chemical Bonds, Covalent Bond Definition Types Polar And What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. Atoms form. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideshare.net
10/26 What are the 3 types of chemical bonds? Part II What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. A. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.totalassignment.com
What are the Different Types of Chemical Bonds? Total assignment Help What Are The Three Types Of Chemical Bonds And How Are They Formed Atoms form bonds because the products are more. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. A bond between two atoms depends upon. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. chemical bonding, any of. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT Types of chemical bonds PowerPoint Presentation, free download What Are The Three Types Of Chemical Bonds And How Are They Formed types of chemical bonds not all chemical bonds form in the same way as the bonds in water. Atoms form bonds because the products are more. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. A bond between two atoms depends upon. the main types of chemical bonds are ionic bond,. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From slideplayer.com
Chemical Bonds Chemical bonds are strong electrostatic forces holding What Are The Three Types Of Chemical Bonds And How Are They Formed A bond between two atoms depends upon. There are actually three different types of. A covalent bond is formed when atoms share valence electrons. An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. the two main types of bonds formed between atoms are ionic bonds and covalent. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From studylib.net
Types of Chemical Bonds What Are The Three Types Of Chemical Bonds And How Are They Formed the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds are described as having different strengths: An ionic bond is formed when one atom accepts or donates one or more of its valence electrons to another atom. chemical bonds form when electrons can be simultaneously close to two. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.studypool.com
SOLUTION Types of chemical bonds Studypool What Are The Three Types Of Chemical Bonds And How Are They Formed Atoms form bonds because the products are more. all models of chemical bonding have three common features: the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood.. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.youtube.com
Chemistry Lesson 31 Different Types of Chemical Bonds YouTube What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds are described as having different strengths: A bond between two atoms depends upon. There are strong bonds or primary bonds such as covalent, ionic and metallic bonds,. the two main types of bonds formed between atoms are ionic bonds and covalent bonds. chemical bonds form when electrons can be simultaneously close to two or more. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From slideplayer.com
A. Types of Chemical Bonds ppt download What Are The Three Types Of Chemical Bonds And How Are They Formed chemical bonds form when electrons can be simultaneously close to two or more nuclei, but beyond this, there is no simple, easily understood. A bond between two atoms depends upon. types of chemical bonds not all chemical bonds form in the same way as the bonds in water. An ionic bond is formed when one atom accepts or. What Are The Three Types Of Chemical Bonds And How Are They Formed.
From www.slideserve.com
PPT 8.1 Chemical Bonds, Lewis Symbols, and the Octet Rule. PowerPoint What Are The Three Types Of Chemical Bonds And How Are They Formed A bond between two atoms depends upon. chemical bonding, any of the interactions that account for the association of atoms into molecules, ions, crystals, and other. A covalent bond is formed when atoms share valence electrons. the main types of chemical bonds are ionic bond, covalent bond, hydrogen bond, and metallic bond [1,2]. chemical bonds form when. What Are The Three Types Of Chemical Bonds And How Are They Formed.