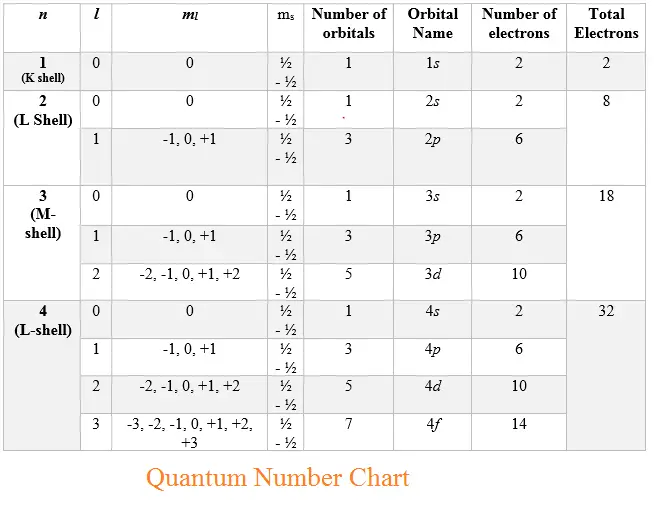
What Is L In Chemistry Quantum Numbers . The first three quantum numbers. Unlike the description of early models of the. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital.
from physicscatalyst.com
There are four quantum numbers that we are going to discuss: Unlike the description of early models of the. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The first three quantum numbers. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. An electron in an atom is completely described by four quantum numbers: N, l, m l, and m s.
Quantum Numbers Chart physicscatalyst's Blog
What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. N, l, m l, and m s. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. An electron in an atom is completely described by four quantum numbers: Unlike the description of early models of the. The first three quantum numbers.
From www.slideserve.com
PPT Quantum numbers and orbital energies Each atom’s electron has a What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. N, l, m l, and m s. There are four quantum numbers that we are going to discuss: An electron in an atom is completely described by four quantum numbers: The first three quantum numbers. Orbitals may be shaped like a sphere, a dumbbell, or. What Is L In Chemistry Quantum Numbers.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin What Is L In Chemistry Quantum Numbers N, l, m l, and m s. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. The first three quantum numbers. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Unlike the description of early models of the. There are four quantum numbers that. What Is L In Chemistry Quantum Numbers.
From scienceinfo.com
Quantum numbers What Is L In Chemistry Quantum Numbers The first three quantum numbers. Unlike the description of early models of the. An electron in an atom is completely described by four quantum numbers: Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. N,. What Is L In Chemistry Quantum Numbers.
From www.nagwa.com
Vidéo de question Déterminer les nombres quantiques qui représentent What Is L In Chemistry Quantum Numbers The first three quantum numbers. Unlike the description of early models of the. N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: There are four quantum numbers that we are going to discuss: The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The principal. What Is L In Chemistry Quantum Numbers.
From www.pinterest.com
Chemistry Quantum Numbers Teaching chemistry, Chemistry classroom What Is L In Chemistry Quantum Numbers Unlike the description of early models of the. The first three quantum numbers. An electron in an atom is completely described by four quantum numbers: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. There. What Is L In Chemistry Quantum Numbers.
From en.ppt-online.org
Atomic structure and properties. (Chapter 3) online presentation What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: There are four quantum numbers that we are going to discuss: Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. Unlike the description. What Is L In Chemistry Quantum Numbers.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. The first three quantum numbers. N, l, m l, and m s. Unlike the description of early models of the. The principal quantum number, n n, describes the energy of an electron and. What Is L In Chemistry Quantum Numbers.
From www.youtube.com
Quantum numbers by Ujjwal sir class 11th chemistry YouTube What Is L In Chemistry Quantum Numbers An electron in an atom is completely described by four quantum numbers: Unlike the description of early models of the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. There are four quantum numbers that we are going to discuss: N, l, m l, and m s. Orbitals may be shaped like a sphere,. What Is L In Chemistry Quantum Numbers.
From savekhaoyai.blogspot.com
41 quantum number practice worksheet Worksheet Database What Is L In Chemistry Quantum Numbers Unlike the description of early models of the. The first three quantum numbers. There are four quantum numbers that we are going to discuss: The orbital angular momentum quantum number, l, provides information about the shape of an orbital. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. An electron in an atom is completely described by. What Is L In Chemistry Quantum Numbers.
From testbook.com
Understand Quantum Numbers Detailed Explanation with Examples What Is L In Chemistry Quantum Numbers The first three quantum numbers. There are four quantum numbers that we are going to discuss: N, l, m l, and m s. Unlike the description of early models of the. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Orbitals may be shaped like a sphere,. What Is L In Chemistry Quantum Numbers.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation What Is L In Chemistry Quantum Numbers N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. The first three quantum numbers. The orbital. What Is L In Chemistry Quantum Numbers.
From www.slideserve.com
PPT Chapter 7 Periodicity and Atomic Structure PowerPoint What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. N, l, m l, and m s. There are four quantum numbers that we are going to discuss: An electron in an atom is completely described by four quantum numbers: The first three quantum numbers. Orbitals may be shaped like a sphere, a dumbbell, or. What Is L In Chemistry Quantum Numbers.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is L In Chemistry Quantum Numbers There are four quantum numbers that we are going to discuss: An electron in an atom is completely described by four quantum numbers: Unlike the description of early models of the. N, l, m l, and m s. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The first three quantum numbers. The principal. What Is L In Chemistry Quantum Numbers.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. Unlike the description of early models of the. There are four quantum numbers that we are going to discuss: An electron in an atom is completely described by four quantum numbers: The principal. What Is L In Chemistry Quantum Numbers.
From www.youtube.com
Chemistry Lesson 11 Figuring Out the Quantum Numbers YouTube What Is L In Chemistry Quantum Numbers There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Unlike the description of early models of the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. Orbitals may be shaped. What Is L In Chemistry Quantum Numbers.
From www.slideserve.com
PPT Principle Quantum Numbers PowerPoint Presentation ID5519904 What Is L In Chemistry Quantum Numbers Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. Unlike the description of early models of the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. There are four quantum numbers that we are going to discuss: An electron in an atom is completely described by four quantum numbers: The principal. What Is L In Chemistry Quantum Numbers.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The first three quantum numbers. Unlike the description of early models of the. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. N, l, m l, and m s. There are four quantum numbers that we are going to discuss: The principal. What Is L In Chemistry Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is L In Chemistry Quantum Numbers The first three quantum numbers. There are four quantum numbers that we are going to discuss: N, l, m l, and m s. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. Unlike the description of early models of the. An electron in an atom is completely described by four quantum numbers: The principal quantum number, n. What Is L In Chemistry Quantum Numbers.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is L In Chemistry Quantum Numbers N, l, m l, and m s. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. An electron in an. What Is L In Chemistry Quantum Numbers.
From hollyaschemblog.blogspot.com
HollyAsChemBlog Quantum Numbers What Is L In Chemistry Quantum Numbers The first three quantum numbers. Unlike the description of early models of the. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. An electron in an atom is completely described by four quantum numbers: N, l, m l, and m s. The orbital angular momentum quantum number,. What Is L In Chemistry Quantum Numbers.
From socratic.org
What are the four quantum numbers in chemistry? Socratic What Is L In Chemistry Quantum Numbers Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. N, l, m l, and m s. There are four quantum. What Is L In Chemistry Quantum Numbers.
From www.pinterest.ph
Quantum Number definition A number assigned to each electron orbit of What Is L In Chemistry Quantum Numbers The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. There are four quantum numbers that we are going to discuss: Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. Unlike the description of early models of the. The first three quantum numbers. An electron. What Is L In Chemistry Quantum Numbers.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps What Is L In Chemistry Quantum Numbers Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. N, l, m l, and m s. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The first three quantum numbers. Unlike the description of early models of the. An electron in an atom is. What Is L In Chemistry Quantum Numbers.
From tuitiontube.com
What is quantum number what do quantum number determine Tuition Tube What Is L In Chemistry Quantum Numbers N, l, m l, and m s. The first three quantum numbers. Unlike the description of early models of the. There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The orbital angular momentum quantum number, l,. What Is L In Chemistry Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 What Is L In Chemistry Quantum Numbers The first three quantum numbers. Unlike the description of early models of the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. An electron in an atom is completely described by four quantum numbers: There are four quantum numbers that we are going to discuss: N, l, m l, and m s. Orbitals may. What Is L In Chemistry Quantum Numbers.
From www.toppr.com
Two of the three electrons in a lithium atom have quantum numbers (n, l What Is L In Chemistry Quantum Numbers The first three quantum numbers. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. N, l, m l, and m s. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. There are four quantum numbers that we are going to discuss: The orbital angular. What Is L In Chemistry Quantum Numbers.
From chem.libretexts.org
8.3 Quantum Numbers for Electrons Chemistry LibreTexts What Is L In Chemistry Quantum Numbers Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. There are four quantum numbers that we are going to discuss: Unlike the description of early models of the. N, l, m l, and m s. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The first three quantum numbers. An electron. What Is L In Chemistry Quantum Numbers.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. N, l, m l, and m s. Unlike the description of early models of the. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. There are four quantum numbers that we are. What Is L In Chemistry Quantum Numbers.
From www.slideserve.com
PPT Quantum Theory PowerPoint Presentation, free download ID1185278 What Is L In Chemistry Quantum Numbers N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: Unlike the description of early models of the. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Orbitals may be shaped like a sphere, a dumbbell, or a. What Is L In Chemistry Quantum Numbers.
From byjus.com
Quantum Numbers Types of Quantum Numbers Chemistry Byju's What Is L In Chemistry Quantum Numbers The first three quantum numbers. Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. An electron in an atom is completely described by four quantum numbers: N, l, m l, and m s. There are four quantum numbers that we are going to discuss: The orbital angular momentum quantum number, l, provides information about the shape of. What Is L In Chemistry Quantum Numbers.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Is L In Chemistry Quantum Numbers There are four quantum numbers that we are going to discuss: The orbital angular momentum quantum number, l, provides information about the shape of an orbital. Unlike the description of early models of the. The first three quantum numbers. N, l, m l, and m s. The principal quantum number, n n, describes the energy of an electron and the. What Is L In Chemistry Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs What Is L In Chemistry Quantum Numbers An electron in an atom is completely described by four quantum numbers: Orbitals may be shaped like a sphere, a dumbbell, or a cloverleaf. N, l, m l, and m s. The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The first three quantum numbers. The orbital. What Is L In Chemistry Quantum Numbers.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice What Is L In Chemistry Quantum Numbers N, l, m l, and m s. An electron in an atom is completely described by four quantum numbers: There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Orbitals may be shaped like a sphere, a. What Is L In Chemistry Quantum Numbers.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog What Is L In Chemistry Quantum Numbers The orbital angular momentum quantum number, l, provides information about the shape of an orbital. There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. Unlike the description of early models of the. Orbitals may be shaped. What Is L In Chemistry Quantum Numbers.
From www.youtube.com
Quantum numbers Electronic structure of atoms Chemistry Khan What Is L In Chemistry Quantum Numbers There are four quantum numbers that we are going to discuss: The principal quantum number, n n, describes the energy of an electron and the most probable distance of the electron from the. The orbital angular momentum quantum number, l, provides information about the shape of an orbital. The first three quantum numbers. Orbitals may be shaped like a sphere,. What Is L In Chemistry Quantum Numbers.