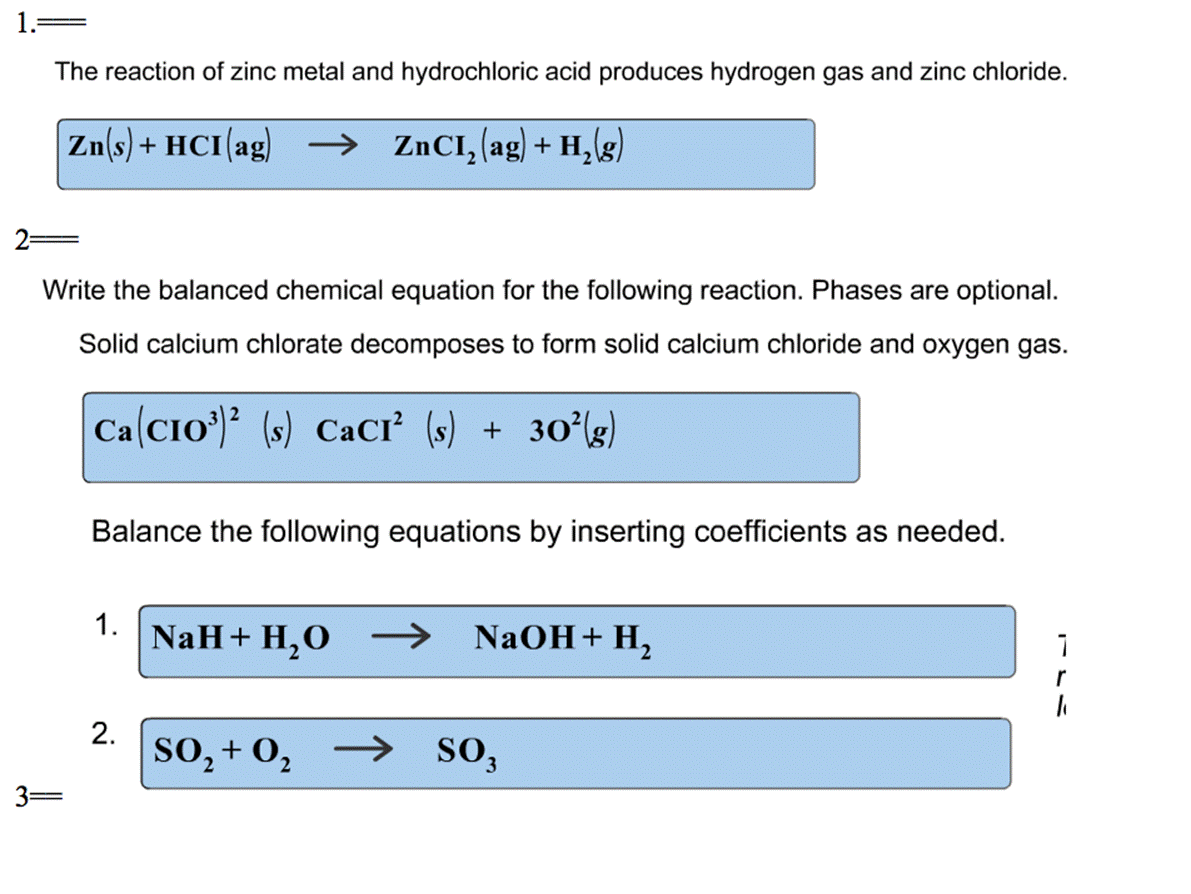
Zinc Equation For Water . In each reaction, hydrogen gas is given off and the. Balance any equation or reaction using this chemical equation balancer! It’s obtained by reacting zinc with sulfur. For example, sodium reacts with water: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Anionic znco 3 has a solubility of 0.21 g/l. Reactions of alkali metals with water. The main form of zinc found in nature, used in luminescent paints and television screens. All the alkali metals react vigorously with cold water. Zinc + water = zinc oxide + dihydrogen. Examples of solubility of zinc compounds are:. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. The alkali metals react with water to produce a metal hydroxide and hydrogen. Acid + metal → salt + hydrogen.
from www.transtutors.com
Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). All the alkali metals react vigorously with cold water. Zinc + water = zinc oxide + dihydrogen. Anionic znco 3 has a solubility of 0.21 g/l. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. Examples of solubility of zinc compounds are:. Acid + metal → salt + hydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. In each reaction, hydrogen gas is given off and the. For example, sodium reacts with water:
(Solved) The Reaction Of Zinc Metal And Hydrochloric Acid Produces
Zinc Equation For Water Balance any equation or reaction using this chemical equation balancer! Reactions of alkali metals with water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Find out what type of reaction occured. Anionic znco 3 has a solubility of 0.21 g/l. In each reaction, hydrogen gas is given off and the. Examples of solubility of zinc compounds are:. Acid + metal → salt + hydrogen. The main form of zinc found in nature, used in luminescent paints and television screens. It’s obtained by reacting zinc with sulfur. Balance any equation or reaction using this chemical equation balancer! Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). The alkali metals react with water to produce a metal hydroxide and hydrogen. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. All the alkali metals react vigorously with cold water. For example, sodium reacts with water:
From www.nagwa.com
Question Video Calculating the Mass of Zinc Carbonate that Will Zinc Equation For Water Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. All the alkali metals react vigorously with cold water. Reactions of alkali metals with water. The main form of zinc found in nature, used in luminescent paints and television screens. Zn + h2o = zno + h2 is a single displacement (substitution) reaction. Zinc Equation For Water.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID Zinc Equation For Water Examples of solubility of zinc compounds are:. The alkali metals react with water to produce a metal hydroxide and hydrogen. The main form of zinc found in nature, used in luminescent paints and television screens. Zinc + water = zinc oxide + dihydrogen. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. Acids will. Zinc Equation For Water.
From www.bartleby.com
Answered When sulfuric acid reacts with zinc… bartleby Zinc Equation For Water In each reaction, hydrogen gas is given off and the. Anionic znco 3 has a solubility of 0.21 g/l. All the alkali metals react vigorously with cold water. For example, sodium reacts with water: Zinc + water = zinc oxide + dihydrogen. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. It’s obtained by. Zinc Equation For Water.
From www.chegg.com
Solved Zinc metal reacts with excess hydrochloric acid to Zinc Equation For Water Find out what type of reaction occured. For example, sodium reacts with water: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Reactions of alkali metals with water. All the alkali metals react vigorously with cold water. In each reaction, hydrogen gas is given off and the. It’s obtained by reacting zinc. Zinc Equation For Water.
From solvedlib.com
1. Part A. Zinc sulfide reacts with oxygen according … SolvedLib Zinc Equation For Water The main form of zinc found in nature, used in luminescent paints and television screens. It’s obtained by reacting zinc with sulfur. Acid + metal → salt + hydrogen. Balance any equation or reaction using this chemical equation balancer! All the alkali metals react vigorously with cold water. For example, sodium reacts with water: Find out what type of reaction. Zinc Equation For Water.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HBr = ZnBr2 + H2 YouTube Zinc Equation For Water The alkali metals react with water to produce a metal hydroxide and hydrogen. All the alkali metals react vigorously with cold water. In each reaction, hydrogen gas is given off and the. Anionic znco 3 has a solubility of 0.21 g/l. Acid + metal → salt + hydrogen. For example, sodium reacts with water: Reactions of alkali metals with water.. Zinc Equation For Water.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Zinc Equation For Water Balance any equation or reaction using this chemical equation balancer! All the alkali metals react vigorously with cold water. Acid + metal → salt + hydrogen. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. It’s obtained by reacting zinc with sulfur. Find out what type of reaction occured. Zinc + water = zinc. Zinc Equation For Water.
From www.numerade.com
SOLVED balanced chemical equation for i) Action of an acid on a base Zinc Equation For Water The alkali metals react with water to produce a metal hydroxide and hydrogen. Zinc + water = zinc oxide + dihydrogen. It’s obtained by reacting zinc with sulfur. For example, sodium reacts with water: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zn + h2o = zno + h2 is a. Zinc Equation For Water.
From www.toppr.com
Write balanced chemical equations for the following word equationZinc Zinc Equation For Water Balance any equation or reaction using this chemical equation balancer! For example, sodium reacts with water: Anionic znco 3 has a solubility of 0.21 g/l. The alkali metals react with water to produce a metal hydroxide and hydrogen. Zinc + water = zinc oxide + dihydrogen. Acid + metal → salt + hydrogen. The main form of zinc found in. Zinc Equation For Water.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc Equation For Water Reactions of alkali metals with water. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. In each reaction, hydrogen gas is given off and the. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.. Zinc Equation For Water.
From www.youtube.com
Zn+H2O=Zn(OH)2+H2 Balanced EquationZinc+Water=Zinc hydroxide+Water Zinc Equation For Water Anionic znco 3 has a solubility of 0.21 g/l. For example, sodium reacts with water: Acid + metal → salt + hydrogen. Reactions of alkali metals with water. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Balance any equation. Zinc Equation For Water.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Equation For Water Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Acid + metal → salt + hydrogen. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Examples of solubility of zinc compounds are:. The alkali metals react with water to produce a metal hydroxide and hydrogen. Reactions of alkali metals. Zinc Equation For Water.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Equation For Water Zinc + water = zinc oxide + dihydrogen. All the alkali metals react vigorously with cold water. Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. The main form of zinc found in nature, used in luminescent paints and television screens. Acids will react with reactive metals, such as magnesium and zinc, to. Zinc Equation For Water.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Zinc Equation For Water Anionic znco 3 has a solubility of 0.21 g/l. Reactions of alkali metals with water. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. Zinc + water = zinc oxide + dihydrogen. Zinc dissolves in water as. Zinc Equation For Water.
From brainly.in
Reaction of zinc with water Brainly.in Zinc Equation For Water Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). For example, sodium reacts with water: Examples of solubility of zinc compounds are:. It’s obtained by reacting zinc with sulfur. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc + water = zinc oxide + dihydrogen. In each reaction,. Zinc Equation For Water.
From tia-jolpblogramsey.blogspot.com
Zinc Carbonate Sulfuric Acid Zinc Equation For Water For example, sodium reacts with water: Reactions of alkali metals with water. The main form of zinc found in nature, used in luminescent paints and television screens. Examples of solubility of zinc compounds are:. The alkali metals react with water to produce a metal hydroxide and hydrogen. All the alkali metals react vigorously with cold water. Zinc dissolves in water. Zinc Equation For Water.
From wiringall.com
Pourbaix Diagram Zinc Zinc Equation For Water Examples of solubility of zinc compounds are:. It’s obtained by reacting zinc with sulfur. Anionic znco 3 has a solubility of 0.21 g/l. For example, sodium reacts with water: The alkali metals react with water to produce a metal hydroxide and hydrogen. The main form of zinc found in nature, used in luminescent paints and television screens. Acids will react. Zinc Equation For Water.
From www.transtutors.com
(Solved) The Reaction Of Zinc Metal And Hydrochloric Acid Produces Zinc Equation For Water Balance any equation or reaction using this chemical equation balancer! The alkali metals react with water to produce a metal hydroxide and hydrogen. Acid + metal → salt + hydrogen. For example, sodium reacts with water: The main form of zinc found in nature, used in luminescent paints and television screens. Acids will react with reactive metals, such as magnesium. Zinc Equation For Water.
From www.numerade.com
SOLVED Question 2 Figure Q2 shows the Pourbaix diagram of zinc metal Zinc Equation For Water Zinc + water = zinc oxide + dihydrogen. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. The alkali metals react with water to produce a metal hydroxide and hydrogen. It’s obtained by reacting zinc with sulfur. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). For example, sodium reacts with. Zinc Equation For Water.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc Equation For Water The main form of zinc found in nature, used in luminescent paints and television screens. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. Anionic znco 3 has a solubility of 0.21 g/l. Find out what type of reaction occured. In each. Zinc Equation For Water.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Equation For Water Examples of solubility of zinc compounds are:. Zinc + water = zinc oxide + dihydrogen. Anionic znco 3 has a solubility of 0.21 g/l. For example, sodium reacts with water: Acid + metal → salt + hydrogen. Find out what type of reaction occured. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. All. Zinc Equation For Water.
From treatybottle13.pythonanywhere.com
Peerless Zinc And Sulfuric Acid Net Ionic Equation Balancing Equations Zinc Equation For Water Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). In each reaction, hydrogen gas is given off and the. Anionic znco 3 has a solubility of 0.21 g/l. Find out what type of reaction occured. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. It’s obtained by reacting zinc. Zinc Equation For Water.
From www.numerade.com
SOLVEDZinc hydroxide is an amphoteric substance. Write equations that Zinc Equation For Water All the alkali metals react vigorously with cold water. Acid + metal → salt + hydrogen. Find out what type of reaction occured. The main form of zinc found in nature, used in luminescent paints and television screens. Zinc + water = zinc oxide + dihydrogen. Anionic znco 3 has a solubility of 0.21 g/l. It’s obtained by reacting zinc. Zinc Equation For Water.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Zinc Equation For Water Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). All the alkali metals react vigorously with cold water. Acid + metal → salt + hydrogen. Zinc + water = zinc oxide + dihydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Reactions of alkali metals with water. Find. Zinc Equation For Water.
From socratic.org
How does zinc form an insoluble zinc hydroxide in water? Socratic Zinc Equation For Water Find out what type of reaction occured. Zinc + water = zinc oxide + dihydrogen. The alkali metals react with water to produce a metal hydroxide and hydrogen. All the alkali metals react vigorously with cold water. Acid + metal → salt + hydrogen. Anionic znco 3 has a solubility of 0.21 g/l. Zinc dissolves in water as znoh +. Zinc Equation For Water.
From www.youtube.com
Is ZnCl2 acidic, basic, or neutral (dissolved in water)? YouTube Zinc Equation For Water Reactions of alkali metals with water. Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. It’s obtained by reacting zinc with sulfur. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. In each reaction, hydrogen gas is given off and the. For example, sodium reacts with. Zinc Equation For Water.
From www.slideserve.com
PPT Zn Solubility PowerPoint Presentation, free download ID3226993 Zinc Equation For Water In each reaction, hydrogen gas is given off and the. For example, sodium reacts with water: Zn + h2o = zno + h2 is a single displacement (substitution) reaction where one. Anionic znco 3 has a solubility of 0.21 g/l. Balance any equation or reaction using this chemical equation balancer! Reactions of alkali metals with water. Zinc dissolves in water. Zinc Equation For Water.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Equation For Water Acid + metal → salt + hydrogen. The main form of zinc found in nature, used in luminescent paints and television screens. Balance any equation or reaction using this chemical equation balancer! Reactions of alkali metals with water. The alkali metals react with water to produce a metal hydroxide and hydrogen. For example, sodium reacts with water: Anionic znco 3. Zinc Equation For Water.
From www.youtube.com
H2SO4+Zn=ZnSO4+H2 Balanced EquationSulphuric acid+Zinc=Zinc sulphate Zinc Equation For Water All the alkali metals react vigorously with cold water. Examples of solubility of zinc compounds are:. Anionic znco 3 has a solubility of 0.21 g/l. Find out what type of reaction occured. For example, sodium reacts with water: In each reaction, hydrogen gas is given off and the. The alkali metals react with water to produce a metal hydroxide and. Zinc Equation For Water.
From www.youtube.com
Solubility equilibrium Ksp zinc hydroxide YouTube Zinc Equation For Water Reactions of alkali metals with water. Zinc + water = zinc oxide + dihydrogen. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! It’s obtained by reacting zinc with sulfur. The main form of zinc. Zinc Equation For Water.
From www.numerade.com
SOLVEDBalance the equation for the reaction in which hot, concentrated Zinc Equation For Water It’s obtained by reacting zinc with sulfur. The alkali metals react with water to produce a metal hydroxide and hydrogen. All the alkali metals react vigorously with cold water. Zinc + water = zinc oxide + dihydrogen. Anionic znco 3 has a solubility of 0.21 g/l. Find out what type of reaction occured. Zinc dissolves in water as znoh +. Zinc Equation For Water.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Equation For Water The alkali metals react with water to produce a metal hydroxide and hydrogen. Examples of solubility of zinc compounds are:. It’s obtained by reacting zinc with sulfur. Reactions of alkali metals with water. Acid + metal → salt + hydrogen. Anionic znco 3 has a solubility of 0.21 g/l. Zn + h2o = zno + h2 is a single displacement. Zinc Equation For Water.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Equation For Water For example, sodium reacts with water: The main form of zinc found in nature, used in luminescent paints and television screens. Zinc + water = zinc oxide + dihydrogen. Reactions of alkali metals with water. Examples of solubility of zinc compounds are:. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. In. Zinc Equation For Water.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Equation For Water Find out what type of reaction occured. Anionic znco 3 has a solubility of 0.21 g/l. For example, sodium reacts with water: Reactions of alkali metals with water. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Zinc + water = zinc oxide + dihydrogen. Zn + h2o = zno + h2. Zinc Equation For Water.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Equation For Water All the alkali metals react vigorously with cold water. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Reactions of alkali metals with water. Examples of solubility of zinc compounds are:. Balance any equation or reaction using this chemical equation balancer! The main form of zinc found in nature, used in luminescent paints and television screens. Zn. Zinc Equation For Water.