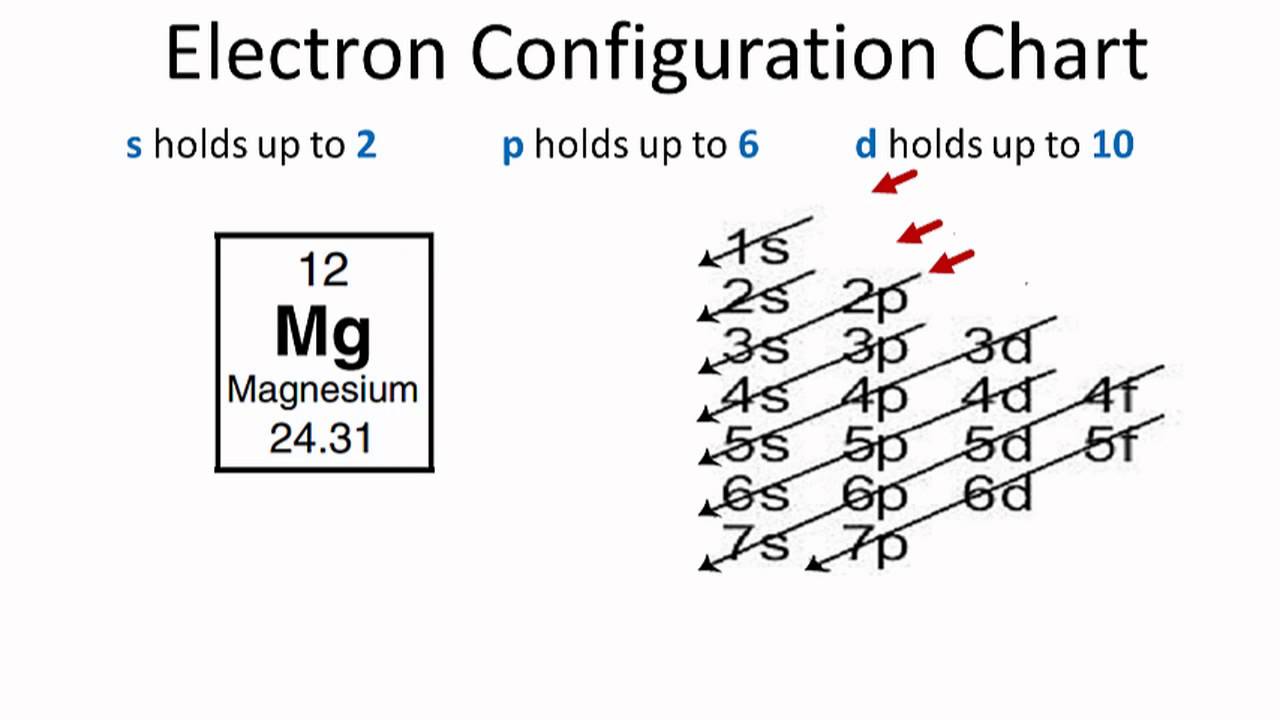
Magnesium Ion Configuration . Magnesium has 12 electrons arranged in three energy levels. Its compounds are widely used in. We’ll also look at why magnesium forms a 2+ ion and. Both atoms have a filled s. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. In this video we will write the electron configuration for mg 2+, the magnesium ion.
from periodictable.me
Both atoms have a filled s. Magnesium has 12 electrons arranged in three energy levels. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Its compounds are widely used in. In this video we will write the electron configuration for mg 2+, the magnesium ion. We’ll also look at why magnesium forms a 2+ ion and. The ground state electron configuration of magnesium is important in understanding its chemical properties. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².
Magnesium Electron Configuration (Mg) with Orbital Diagram
Magnesium Ion Configuration Magnesium has 12 electrons arranged in three energy levels. Its compounds are widely used in. Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In this video we will write the electron configuration for mg 2+, the magnesium ion. We’ll also look at why magnesium forms a 2+ ion and. Both atoms have a filled s. The ground state electron configuration of magnesium is important in understanding its chemical properties. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Ion Configuration Both atoms have a filled s. We’ll also look at why magnesium forms a 2+ ion and. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Magnesium has 12 electrons arranged in three energy levels. The ground state electron configuration of magnesium is. Magnesium Ion Configuration.
From guidemanualeruptivity.z14.web.core.windows.net
Magnesium Electron Configuration Diagram Magnesium Ion Configuration Both atoms have a filled s. Magnesium has 12 electrons arranged in three energy levels. Its compounds are widely used in. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In this video we will write the electron configuration for mg 2+, the magnesium ion. The ground state electron configuration of magnesium is important in understanding its chemical properties.. Magnesium Ion Configuration.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Configuration In this video we will write the electron configuration for mg 2+, the magnesium ion. Magnesium has 12 electrons arranged in three energy levels. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. We’ll also look at why magnesium forms a 2+ ion. Magnesium Ion Configuration.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Configuration Its compounds are widely used in. Magnesium has 12 electrons arranged in three energy levels. We’ll also look at why magnesium forms a 2+ ion and. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. Both atoms have a filled s. In order to write. Magnesium Ion Configuration.
From valenceelectrons.com
Electron Configuration for Magnesium(Mg, Mg2+ ion) Magnesium Ion Configuration Its compounds are widely used in. We’ll also look at why magnesium forms a 2+ ion and. Magnesium has 12 electrons arranged in three energy levels. The ground state electron configuration of magnesium is important in understanding its chemical properties. In this video we will write the electron configuration for mg 2+, the magnesium ion. Both atoms have a filled. Magnesium Ion Configuration.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Configuration Both atoms have a filled s. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). In this video we will write the electron configuration for mg 2+, the magnesium ion. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a. Magnesium Ion Configuration.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion Configuration Both atoms have a filled s. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Its compounds are widely used in. In order to write the mg electron configuration we first need to. Magnesium Ion Configuration.
From celbncbc.blob.core.windows.net
Magnesium Ion Number Of Electrons at Joan Harbert blog Magnesium Ion Configuration In this video we will write the electron configuration for mg 2+, the magnesium ion. Magnesium has 12 electrons arranged in three energy levels. The ground state electron configuration of magnesium is important in understanding its chemical properties. Its compounds are widely used in. Both atoms have a filled s. The alkaline earth metal magnesium (atomic number 12), with its. Magnesium Ion Configuration.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Configuration In this video we will write the electron configuration for mg 2+, the magnesium ion. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. The ground state electron configuration of magnesium is important in understanding its chemical properties. In order to write the. Magnesium Ion Configuration.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Ion Configuration The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Both atoms have a filled s. We’ll also look at why magnesium forms a 2+ ion and. The ground state electron configuration of magnesium is important in understanding its chemical properties. The electron configuration. Magnesium Ion Configuration.
From exoilgobw.blob.core.windows.net
Magnesium Ion Explanation at Helen Weeks blog Magnesium Ion Configuration The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Both atoms have a filled s. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Its compounds are. Magnesium Ion Configuration.
From exoilgobw.blob.core.windows.net
Magnesium Ion Explanation at Helen Weeks blog Magnesium Ion Configuration The ground state electron configuration of magnesium is important in understanding its chemical properties. Its compounds are widely used in. In this video we will write the electron configuration for mg 2+, the magnesium ion. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons).. Magnesium Ion Configuration.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion Configuration The ground state electron configuration of magnesium is important in understanding its chemical properties. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Its compounds are widely used in. Magnesium has 12 electrons arranged. Magnesium Ion Configuration.
From www.youtube.com
(a). Write down the electronic configuration of (i) magnesium atom, and Magnesium Ion Configuration In this video we will write the electron configuration for mg 2+, the magnesium ion. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Its compounds are widely used in. We’ll also look at. Magnesium Ion Configuration.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Configuration The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Its compounds are widely used in. Magnesium has 12 electrons arranged in three energy levels. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Both atoms have a filled s. The ground state electron. Magnesium Ion Configuration.
From www.thesciencehive.co.uk
Atomic Structure and Electron Configuration (AQA) — the science hive Magnesium Ion Configuration The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Both atoms have a filled s. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In this video we will write the electron configuration for mg 2+, the magnesium ion. The ground state. Magnesium Ion Configuration.
From enginedatanichered.z21.web.core.windows.net
Magnesium Atom Diagram Magnesium Ion Configuration The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The ground state electron configuration of magnesium is important in understanding its chemical properties. We’ll also look at why magnesium forms a 2+ ion and.. Magnesium Ion Configuration.
From www.youtube.com
Magnesium ionwhat you need to know. Electron Configuration YouTube Magnesium Ion Configuration Both atoms have a filled s. In this video we will write the electron configuration for mg 2+, the magnesium ion. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². We’ll also look. Magnesium Ion Configuration.
From brainly.com
What is the electron configuration for the Magnesium ion? Magnesium Ion Configuration The ground state electron configuration of magnesium is important in understanding its chemical properties. We’ll also look at why magnesium forms a 2+ ion and. Its compounds are widely used in. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. In this video we will write the electron configuration for. Magnesium Ion Configuration.
From valenceelectrons.com
Electron Configuration for Magnesium and ion(Mg2+) Magnesium Ion Configuration We’ll also look at why magnesium forms a 2+ ion and. Both atoms have a filled s. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The ground state electron configuration of magnesium is. Magnesium Ion Configuration.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Configuration We’ll also look at why magnesium forms a 2+ ion and. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Its compounds are widely used in. Both atoms have a filled s. In order to write the mg electron configuration we first need. Magnesium Ion Configuration.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Ion Configuration Its compounds are widely used in. In this video we will write the electron configuration for mg 2+, the magnesium ion. We’ll also look at why magnesium forms a 2+ ion and. The ground state electron configuration of magnesium is important in understanding its chemical properties. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The alkaline earth metal. Magnesium Ion Configuration.
From mungfali.com
Magnesium Ion Electron Configuration Magnesium Ion Configuration In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Both atoms have a filled s. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. The ground state. Magnesium Ion Configuration.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Ion Configuration In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. Magnesium has 12 electrons arranged in three energy levels. The alkaline. Magnesium Ion Configuration.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Ion Configuration The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Its compounds are widely used in. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In this video we will write the electron configuration for mg 2+, the magnesium ion. Magnesium has 12. Magnesium Ion Configuration.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Ion Configuration In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons). Both atoms have a filled s. Magnesium has 12 electrons arranged in three energy levels. The ground state electron configuration of magnesium is important in understanding its chemical properties. The electron configuration of magnesium is. Magnesium Ion Configuration.
From uzabytaodi.blogspot.com
41 orbital diagram of magnesium Wiring Diagrams Manual Magnesium Ion Configuration The ground state electron configuration of magnesium is important in understanding its chemical properties. Both atoms have a filled s. In this video we will write the electron configuration for mg 2+, the magnesium ion. In order to write the mg electron configuration we first need to know the number of electrons for the mg atom (there are 12 electrons).. Magnesium Ion Configuration.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion Configuration In this video we will write the electron configuration for mg 2+, the magnesium ion. Magnesium has 12 electrons arranged in three energy levels. Both atoms have a filled s. The ground state electron configuration of magnesium is important in understanding its chemical properties. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2. Magnesium Ion Configuration.
From circuitlibbottega.z21.web.core.windows.net
Magnesium Dot Diagram Magnesium Ion Configuration Its compounds are widely used in. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. We’ll also look at why magnesium forms a 2+ ion and. The ground state electron configuration of magnesium is important in understanding its chemical properties. In order to. Magnesium Ion Configuration.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Configuration The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. Magnesium has 12 electrons arranged in three energy levels. In this video we will write the electron configuration for mg 2+, the magnesium ion. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons. Magnesium Ion Configuration.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Ion Configuration The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. We’ll also look at why magnesium forms a 2+ ion and. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Both atoms have a filled s. Magnesium has 12 electrons arranged in three. Magnesium Ion Configuration.
From www.youtube.com
Magnésium (Mg) Configuration électronique Formule et schéma de Magnesium Ion Configuration The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its chemical properties. Its compounds are widely used in. Both atoms have a. Magnesium Ion Configuration.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Magnesium Ion Configuration Its compounds are widely used in. Both atoms have a filled s. Magnesium has 12 electrons arranged in three energy levels. We’ll also look at why magnesium forms a 2+ ion and. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. The electron. Magnesium Ion Configuration.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion Configuration In this video we will write the electron configuration for mg 2+, the magnesium ion. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. We’ll also look at why magnesium forms a 2+ ion and. In order to write the mg electron configuration. Magnesium Ion Configuration.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Magnesium Ion Configuration The ground state electron configuration of magnesium is important in understanding its chemical properties. The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [ne]3s 2 configuration, is analogous to its family member beryllium, [he]2s 2. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². We’ll. Magnesium Ion Configuration.