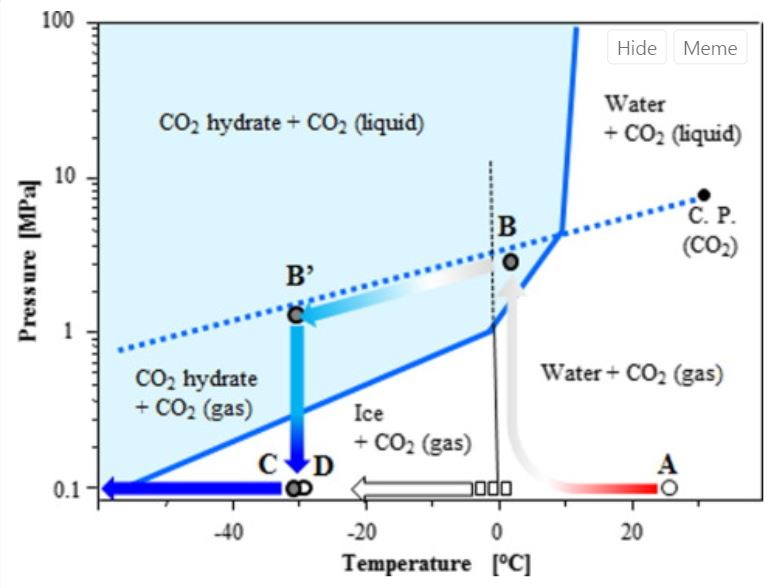
Phase Diagram Water Vs Co2 . Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows:
from ar.inspiredpencil.com
An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. However for other substances, notably water, the line slopes to. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm.
Phase Diagram Of Water Vs Co2
Phase Diagram Water Vs Co2 A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. However for other substances, notably water, the line slopes to. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a.
From www.aiophotoz.com
Phase Diagram Water Vs Co2 Diagram Media Images and Photos finder Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. An explanation of how to interpret the phase diagrams for. Phase Diagram Water Vs Co2.
From www.101diagrams.com
Phase Diagram of CO2 101 Diagrams Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to. A phase diagram of. Phase Diagram Water Vs Co2.
From app.jove.com
Phase Diagrams Carbon Dioxide and Water Phase Diagrams Concept Phase Diagram Water Vs Co2 However for other substances, notably water, the line slopes to. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right.. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. However for other substances, notably water, the line slopes to. Normally the. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing. Phase Diagram Water Vs Co2.
From www.101diagrams.com
CO2 Phase Diagram 101 Diagrams Phase Diagram Water Vs Co2 Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide. Phase Diagram Water Vs Co2.
From www.youtube.com
Phase diagram of water and carbon dioxide explained YouTube Phase Diagram Water Vs Co2 A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. However for other substances, notably water, the line slopes to. Using the phase diagram for carbon. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 However for other substances, notably water, the line slopes to. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas). Phase Diagram Water Vs Co2.
From sagabio.com
Phase Diagram Of Carbon Dioxide Different From Water Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. However for other substances, notably water, the line slopes to. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a.. Phase Diagram Water Vs Co2.
From www.youtube.com
Phase Diagram Terms Explained with Examples (Water and Carbon Dioxide Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). An explanation of how to interpret the phase diagrams for pure substances including carbon. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 However for other substances, notably water, the line slopes to. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. Normally the solid/liquid phase line slopes positively to the right (as. Phase Diagram Water Vs Co2.
From www.researchgate.net
Phase diagram of the watercarbon dioxide mixture. Comparison of Phase Diagram Water Vs Co2 Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: However for other substances, notably water, the line slopes to. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. In contrast. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. However for other substances, notably water, the line slopes to. Normally the solid/liquid phase line slopes positively to the right (as. Phase Diagram Water Vs Co2.
From mavink.com
Diagrama De Fases Del Co2 Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. However for other substances, notably water, the line slopes to. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). A phase diagram of. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. A phase diagram of water is a graphical representation of water’s phases (solid, liquid,. Phase Diagram Water Vs Co2.
From www.chemistrylearner.com
Carbon Dioxide (CO2) Phase Diagram Phase Diagram Water Vs Co2 The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. An explanation of how to interpret the phase diagrams for. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and. Phase Diagram Water Vs Co2.
From www.101diagrams.com
Phase Diagram of CO2 101 Diagrams Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. However for other substances, notably water, the line slopes to. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas). Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. An explanation of how to interpret the phase diagrams for. Phase Diagram Water Vs Co2.
From chemix-chemistry-software.com
Carbon dioxide vs water phase diagrams Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to. A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. In contrast to the phase diagram of water, the phase. Phase Diagram Water Vs Co2.
From dgreen.beauty
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. An explanation of how to interpret the phase diagrams for pure substances. Phase Diagram Water Vs Co2.
From www.pnas.org
Stability of dense liquid carbon dioxide PNAS Phase Diagram Water Vs Co2 In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. However for other substances, notably water, the line slopes to. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). The triple point is. Phase Diagram Water Vs Co2.
From www.slideserve.com
PPT Intermolecular forces PowerPoint Presentation, free download ID Phase Diagram Water Vs Co2 A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to. Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: The triple point is −56.6°c and. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: In contrast to the phase diagram of water, the phase diagram of. Phase Diagram Water Vs Co2.
From www.jove.com
Phase Diagrams Carbon Dioxide and Water Phase Diagrams JoVE Phase Diagram Water Vs Co2 An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. However for other substances, notably water, the line slopes to. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right. The triple point is −56.6°c. Phase Diagram Water Vs Co2.
From sagabio.com
Phase Diagram Of Carbon Dioxide Different From Water Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)). Phase Diagram Water Vs Co2.
From www.researchgate.net
Phase diagram of pure CO2 is shown here. Liquid and gas phases will Phase Diagram Water Vs Co2 An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. However for other substances, notably water, the line slopes to. Using the phase diagram for carbon dioxide provided, we can determine. Phase Diagram Water Vs Co2.
From chem.libretexts.org
Phase Diagrams Chemistry LibreTexts Phase Diagram Water Vs Co2 An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. The triple point. Phase Diagram Water Vs Co2.
From www.numerade.com
SOLVED Below are the phase diagrams for water and carbon dioxide Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. However for other substances, notably water, the line slopes to. In contrast to the phase diagram of water, the phase. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Using the phase diagram for carbon dioxide provided, we can determine that the state of co 2 at each temperature and pressure given are as follows: Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). However for other substances, notably water, the line slopes to. A phase diagram of water is. Phase Diagram Water Vs Co2.
From app.jove.com
Phase Diagrams Carbon Dioxide and Water Phase Diagrams Concept Phase Diagram Water Vs Co2 However for other substances, notably water, the line slopes to. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. In contrast to the phase diagram of water, the phase diagram of co 2 (figure \(\pageindex{3}\)) has a more typical melting curve, sloping up and to the right.. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 Normally the solid/liquid phase line slopes positively to the right (as in the diagram for carbon dioxide below). An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11 atm. In contrast to. Phase Diagram Water Vs Co2.
From ar.inspiredpencil.com
Phase Diagram Of Water Vs Co2 Phase Diagram Water Vs Co2 A phase diagram of water is a graphical representation of water’s phases (solid, liquid, gas) under changing temperature and pressure, while a. An explanation of how to interpret the phase diagrams for pure substances including carbon dioxide and water. The triple point is −56.6°c and 5.11 atm, which means that liquid co 2 cannot exist at pressures lower than 5.11. Phase Diagram Water Vs Co2.