Autoclave Guidelines In Pharmaceutical Industry . this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. — this article has procedure for autoclave validation including steam penetration, heat distribution and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for.
from www.pharmasciences.in
— this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for.
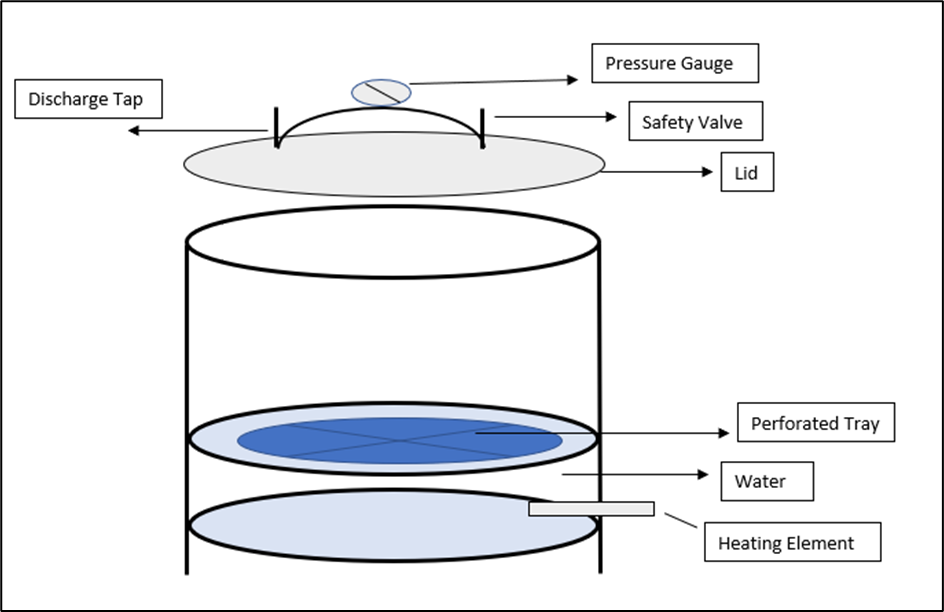
Basic Components with Autoclave diagram PharmaSciences
Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when.
From lowepowerlab.ucdavis.edu
Autoclave protocol protocols Autoclave Guidelines In Pharmaceutical Industry who good manufacturing practices for sterile pharmaceutical products. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. — this article has procedure for autoclave validation including steam penetration, heat distribution and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards. Autoclave Guidelines In Pharmaceutical Industry.
From www.zirbus.com
Large Autoclaves for Production Made in Germany Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards. Autoclave Guidelines In Pharmaceutical Industry.
From solutionpharmacy.in
Physical Methods of Sterilization Solution Parmacy Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. — this article has procedure for autoclave validation including steam penetration, heat distribution. Autoclave Guidelines In Pharmaceutical Industry.
From www.linkedin.com
How to Choose Pharmaceutical Autoclaves Autoclave Guidelines In Pharmaceutical Industry who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. — this article has procedure for autoclave validation including steam penetration, heat distribution and.. Autoclave Guidelines In Pharmaceutical Industry.
From consteril.com
Standard Autoclave Procedures (SOPs) Guide + Template Autoclave Guidelines In Pharmaceutical Industry this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. — this article has procedure for autoclave validation including steam penetration, heat distribution and.. Autoclave Guidelines In Pharmaceutical Industry.
From www.scribd.com
STERIS Century Series Autoclave User Guidelines & Standard Operating Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical. Autoclave Guidelines In Pharmaceutical Industry.
From www.dreamstime.com
Pharmaceutical Factory. Worker Operating Autoclave for Medicine Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. — this article has procedure for autoclave validation including steam penetration, heat distribution and.. Autoclave Guidelines In Pharmaceutical Industry.
From mavink.com
Autoclave Schematic Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards. Autoclave Guidelines In Pharmaceutical Industry.
From www.slideshare.net
Equitron Praano Series Autoclaves for Pharma, R&D, QA, QC, Micro Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. — this article has. Autoclave Guidelines In Pharmaceutical Industry.
From www.syntegon.com
Autoclaves from SBM support biopharmaceutical production facilities Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. who good manufacturing practices for sterile pharmaceutical products. — access to medicines and health products (mhp), health product policy and standards (hps),. Autoclave Guidelines In Pharmaceutical Industry.
From thebiologynotes.com
Laboratory Autoclave Definition, Principle, Parts, Types, Uses Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp),. Autoclave Guidelines In Pharmaceutical Industry.
From www.microlit.us
What is Autoclaving, its Process, Meaning, Autoclave Sterilization Autoclave Guidelines In Pharmaceutical Industry who good manufacturing practices for sterile pharmaceutical products. — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when.. Autoclave Guidelines In Pharmaceutical Industry.
From lifetechqa.blogspot.com
AUTOCLAVE VALIDATION (VERTICAL) Pharmaceutical Guidelines Autoclave Guidelines In Pharmaceutical Industry who good manufacturing practices for sterile pharmaceutical products. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active. Autoclave Guidelines In Pharmaceutical Industry.
From studylib.net
a guideline for the safe use of autoclaves Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards. Autoclave Guidelines In Pharmaceutical Industry.
From www.pharmasciences.in
Basic Components with Autoclave diagram PharmaSciences Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. who good manufacturing practices. Autoclave Guidelines In Pharmaceutical Industry.
From microbeonline.com
Autoclave Sterilization Principle, Procedure, Types, Uses • Microbe Online Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. — this article has. Autoclave Guidelines In Pharmaceutical Industry.
From www.scribd.com
Autoclave Machine Uses, Guidelines & Cost Knowledge Center PDF Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts. Autoclave Guidelines In Pharmaceutical Industry.
From ritmindustry.com
Laboratory autoclave / horizontal / for the pharmaceutical industry Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has. Autoclave Guidelines In Pharmaceutical Industry.
From www.lihpao.com
How Does an Autoclave Work? A StepbyStep Guide to Sterilization The Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has procedure for autoclave validation including steam penetration, heat distribution and. this guidance pertains to current good manufacturing practice. Autoclave Guidelines In Pharmaceutical Industry.
From www.alamy.com
pharmaceutical factory woman worker operating autoclave for medicine Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices. Autoclave Guidelines In Pharmaceutical Industry.
From www.youtube.com
STERILIZATION PART4 AUTOCLAVE PRINCIPLE MECHANISM WORKING Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. who good manufacturing practices. Autoclave Guidelines In Pharmaceutical Industry.
From www.medicalexpo.com
Autoclave for the pharmaceutical industry ETO RSD Engineering Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts. Autoclave Guidelines In Pharmaceutical Industry.
From www.dreamstime.com
Pharmaceutical Factory. Worker Operating Autoclave for Medicine Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards. Autoclave Guidelines In Pharmaceutical Industry.
From ritmindustry.com
Laboratory autoclave / horizontal / for the pharmaceutical industry Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and.. Autoclave Guidelines In Pharmaceutical Industry.
From manualzz.com
NU Autoclave Guidelines Manualzz Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has procedure for autoclave validation including steam penetration, heat distribution and. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. who good manufacturing practices for sterile pharmaceutical products.. Autoclave Guidelines In Pharmaceutical Industry.
From www.alphascientific.ca
Autoclave use in the pharmaceutical industry Canada Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active. Autoclave Guidelines In Pharmaceutical Industry.
From www.duralinesystems.com
AutoclaveTraining Onsite Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp),. Autoclave Guidelines In Pharmaceutical Industry.
From consteril.com
Autoclave Installation for Leading Biopharmaceutical Company Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has procedure for autoclave validation including steam penetration, heat distribution and. — access to medicines and health products (mhp),. Autoclave Guidelines In Pharmaceutical Industry.
From microbeonline.com
Autoclave Principle, Procedure, Types and Uses Learn Microbiology Online Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. — this article has procedure for autoclave validation including steam penetration, heat distribution and. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. who good manufacturing practices for sterile pharmaceutical. Autoclave Guidelines In Pharmaceutical Industry.
From lsi1.com
Understanding Saturated Steam Pharmaceutical Autoclave High Flexibility Autoclave Guidelines In Pharmaceutical Industry who good manufacturing practices for sterile pharmaceutical products. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — access to medicines and health products (mhp), health product policy and standards (hps),. Autoclave Guidelines In Pharmaceutical Industry.
From moonmed.com
How Does an Autoclave Work How to Use Autoclaves? Autoclave Guidelines In Pharmaceutical Industry guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has procedure for autoclave validation including steam penetration, heat distribution and. who good manufacturing practices for sterile pharmaceutical products. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and.. Autoclave Guidelines In Pharmaceutical Industry.
From ritmindustry.com
Laboratory autoclave / horizontal / for the pharmaceutical industry Autoclave Guidelines In Pharmaceutical Industry this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. who good manufacturing practices for sterile pharmaceutical products. — this article has procedure for autoclave validation including steam penetration, heat distribution and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards. Autoclave Guidelines In Pharmaceutical Industry.
From jewelprecision.com
What Is The Autoclave Process? Learn Here! Autoclave Guidelines In Pharmaceutical Industry — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected for sterile finished products, sterile active substances, sterile excipients and. — this article has. Autoclave Guidelines In Pharmaceutical Industry.
From medsolut.com
Validation of the autoclave procedure and guidelines MedSolut Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211) when. guidance is provided on the documentation expected. Autoclave Guidelines In Pharmaceutical Industry.
From www.vedantu.com
Uses of Autoclave Learn Important Terms and Concepts Autoclave Guidelines In Pharmaceutical Industry — this article has procedure for autoclave validation including steam penetration, heat distribution and. who good manufacturing practices for sterile pharmaceutical products. — access to medicines and health products (mhp), health product policy and standards (hps), norms and standards for. this guidance pertains to current good manufacturing practice (cgmp) regulations (21 cfr parts 210 and 211). Autoclave Guidelines In Pharmaceutical Industry.