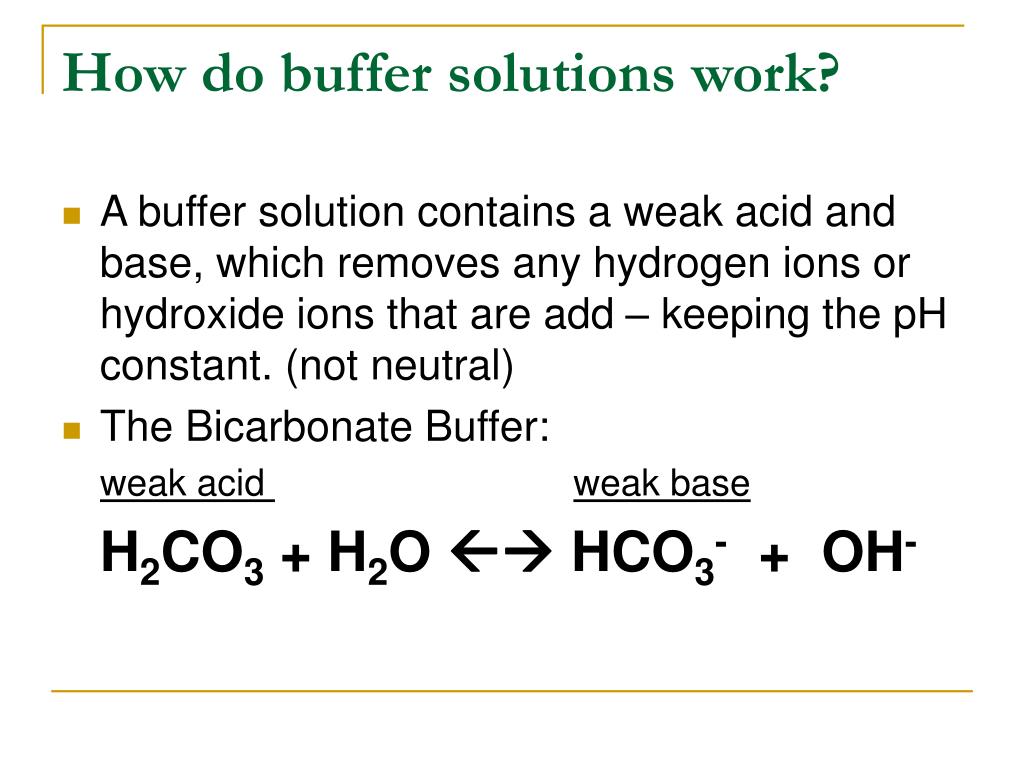
What Is A Buffer Solution Made Of . A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. It consists of a solution of a weak acid and its.
from www.slideserve.com
A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. It consists of a solution of a weak acid and its. Buffer solutions resist a change in ph. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.
PPT Buffers PowerPoint Presentation, free download ID6787517
What Is A Buffer Solution Made Of What is a buffer solution? It consists of a solution of a weak acid and its. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 What Is A Buffer Solution Made Of A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. It consists of a solution of a weak acid and its. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.. What Is A Buffer Solution Made Of.
From chemistryguru.com.sg
What is a Buffer Solution? What Is A Buffer Solution Made Of A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It consists of a solution of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that maintains. What Is A Buffer Solution Made Of.
From ceirptus.blob.core.windows.net
What Is Buffer Solution In Chemistry at Elizabeth Olsen blog What Is A Buffer Solution Made Of In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.. What Is A Buffer Solution Made Of.
From golifescience.com
Buffer Solution definition, 4 Types and Basic Calculations What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A. What Is A Buffer Solution Made Of.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution What Is A Buffer Solution Made Of A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that maintains the stability of a system’s ph level when adding small. What Is A Buffer Solution Made Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Solution Made Of What is a buffer solution? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of. What Is A Buffer Solution Made Of.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions What Is A Buffer Solution Made Of A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. What is a buffer solution? In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions. What Is A Buffer Solution Made Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph. What Is A Buffer Solution Made Of.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. What is a buffer solution? Buffer solutions resist a change in ph. A buffer solution is one which resists changes in ph when small quantities. What Is A Buffer Solution Made Of.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is A Buffer Solution Made Of A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of. What Is A Buffer Solution Made Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Solution Made Of It consists of a solution of a weak acid and its. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base.. What Is A Buffer Solution Made Of.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It consists of a solution of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph when small quantities of. What Is A Buffer Solution Made Of.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition. What Is A Buffer Solution Made Of.
From ceirptus.blob.core.windows.net
What Is Buffer Solution In Chemistry at Elizabeth Olsen blog What Is A Buffer Solution Made Of A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. What is a buffer solution? Buffer solutions resist a change in ph. A buffer is a. What Is A Buffer Solution Made Of.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Solution Made Of A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? In chemistry, the definition. What Is A Buffer Solution Made Of.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation What Is A Buffer Solution Made Of It consists of a solution of a weak acid and its. Buffer solutions resist a change in ph. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. What is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an. What Is A Buffer Solution Made Of.
From www.youtube.com
Buffer Solutions YouTube What Is A Buffer Solution Made Of In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph. What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon. What Is A Buffer Solution Made Of.
From riaquueta45.blogspot.com
What Is Meant By Buffer Solution Give An Example Of Basic Buffer What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a solution. What Is A Buffer Solution Made Of.
From www.chemicals.co.uk
What Is A Buffer Solution? The Chemistry Blog What Is A Buffer Solution Made Of A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize. What Is A Buffer Solution Made Of.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide What Is A Buffer Solution Made Of A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. What is a buffer solution? It consists of a solution of a weak acid and its. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture. What Is A Buffer Solution Made Of.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. It is able to neutralize small amounts of added. What Is A Buffer Solution Made Of.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download What Is A Buffer Solution Made Of A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph. It is able to neutralize small amounts of added acid or base, thus. It consists of a solution of a weak acid and its. A mixture of a weak acid and its conjugate base. What Is A Buffer Solution Made Of.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is A Buffer Solution Made Of A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. Buffer solutions resist a change in ph. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that maintains the stability. What Is A Buffer Solution Made Of.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Is A Buffer Solution Made Of What is a buffer solution? A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. It consists of a solution of a weak acid and its. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer. What Is A Buffer Solution Made Of.
From www.youtube.com
What is Buffer Solution Types of Buffer Solution Acidic Buffer What Is A Buffer Solution Made Of It is able to neutralize small amounts of added acid or base, thus. It consists of a solution of a weak acid and its. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A mixture of a weak acid and its conjugate base (or a mixture of a weak. What Is A Buffer Solution Made Of.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is A Buffer Solution Made Of What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. Buffer solutions resist. What Is A Buffer Solution Made Of.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Is A Buffer Solution Made Of What is a buffer solution? Buffer solutions resist a change in ph. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is A Buffer Solution Made Of.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer Solution Made Of In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer solution is one which resists changes in ph when small quantities of. What Is A Buffer Solution Made Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A mixture of a weak acid. What Is A Buffer Solution Made Of.
From ceirptus.blob.core.windows.net
What Is Buffer Solution In Chemistry at Elizabeth Olsen blog What Is A Buffer Solution Made Of A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. It is able to neutralize small amounts of added acid or base, thus. A. What Is A Buffer Solution Made Of.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer Solution Made Of In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. Buffer solutions resist a change in ph. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A mixture of a weak acid and its conjugate. What Is A Buffer Solution Made Of.
From ceirptus.blob.core.windows.net
What Is Buffer Solution In Chemistry at Elizabeth Olsen blog What Is A Buffer Solution Made Of Buffer solutions resist a change in ph. It consists of a solution of a weak acid and its. In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and. What Is A Buffer Solution Made Of.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Solution Made Of It consists of a solution of a weak acid and its. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. What is a buffer solution? It is able to neutralize small amounts of added acid or base, thus. A buffer. What Is A Buffer Solution Made Of.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Is A Buffer Solution Made Of It consists of a solution of a weak acid and its. It is able to neutralize small amounts of added acid or base, thus. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are. A buffer is a solution that maintains the stability of a system’s ph level when adding. What Is A Buffer Solution Made Of.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is A Buffer Solution Made Of In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an acid or a base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions resist a change in ph. It is able to neutralize small amounts of added acid. What Is A Buffer Solution Made Of.