What Is The Solvent Polarity . Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. Because of the shape of the. because water is polar, substances that are polar or ionic will dissolve in it. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. in this explainer, we will learn how to describe polar and nonpolar solvents. 23 rows solvent polarity. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. Solvents are generally classified by the polarity, and considered either polar or non. Solubility can be defined as the tendency. This creates a slight imbalance of the. polar solvents are best for dissolving polar reactants (such as ions); today, we will explore how polar organic solvents are, and will test what mixes with what.
from www.chemistryworld.com
This creates a slight imbalance of the. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. polar solvents are best for dissolving polar reactants (such as ions); today, we will explore how polar organic solvents are, and will test what mixes with what. 23 rows solvent polarity. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. in this explainer, we will learn how to describe polar and nonpolar solvents. Because of the shape of the. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Solvents are generally classified by the polarity, and considered either polar or non.
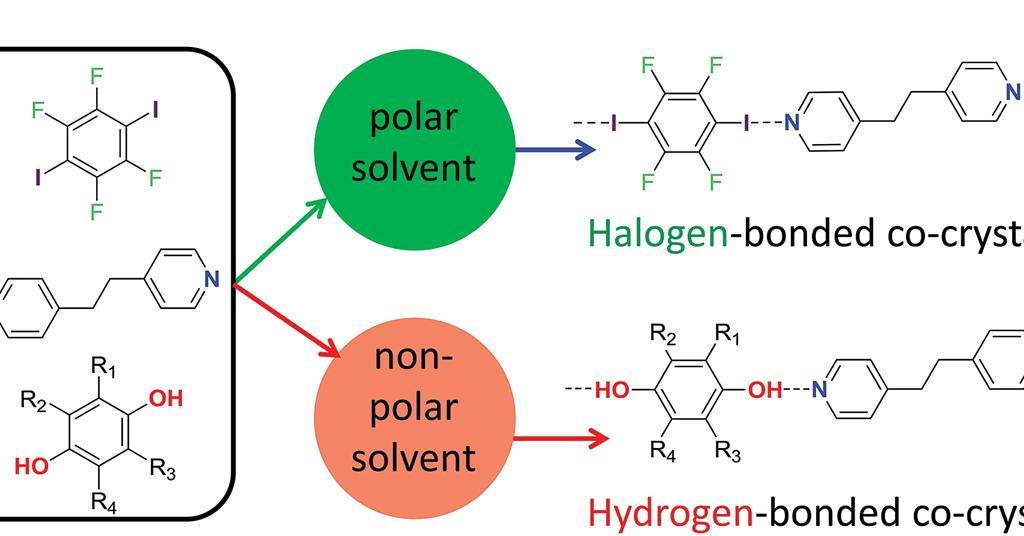
Polar solvents promote halogen bonds over hydrogen ones Research
What Is The Solvent Polarity Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. because water is polar, substances that are polar or ionic will dissolve in it. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. today, we will explore how polar organic solvents are, and will test what mixes with what. 23 rows solvent polarity. Because of the shape of the. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. in this explainer, we will learn how to describe polar and nonpolar solvents. polar solvents are best for dissolving polar reactants (such as ions); Solvents are generally classified by the polarity, and considered either polar or non. Solubility can be defined as the tendency. This creates a slight imbalance of the.
From www.slideserve.com
PPT Substitution Reactions of Alkyl Halides Chapter 8 PowerPoint What Is The Solvent Polarity polar solvents are best for dissolving polar reactants (such as ions); 23 rows solvent polarity. today, we will explore how polar organic solvents are, and will test what mixes with what. Solvents are generally classified by the polarity, and considered either polar or non. Solubility can be defined as the tendency. solvent polarity refers to the. What Is The Solvent Polarity.
From brainly.in
What is a polar solvent ? Brainly.in What Is The Solvent Polarity polar solvents are best for dissolving polar reactants (such as ions); because water is polar, substances that are polar or ionic will dissolve in it. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Because of the shape of the. Solubility can be defined as. What Is The Solvent Polarity.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID What Is The Solvent Polarity the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. 23 rows solvent polarity. polar solvents are best for dissolving polar reactants (such as ions); because water. What Is The Solvent Polarity.
From www.chemistryworld.com
Polar solvents promote halogen bonds over hydrogen ones Research What Is The Solvent Polarity in this explainer, we will learn how to describe polar and nonpolar solvents. 23 rows solvent polarity. Because of the shape of the. This creates a slight imbalance of the. because water is polar, substances that are polar or ionic will dissolve in it. solvent polarity refers to the ability of a solvent to dissolve polar. What Is The Solvent Polarity.
From www.youtube.com
Chapter 08 05 Water A Polar Solvent YouTube What Is The Solvent Polarity polar solvents are best for dissolving polar reactants (such as ions); because water is polar, substances that are polar or ionic will dissolve in it. 23 rows solvent polarity. in this explainer, we will learn how to describe polar and nonpolar solvents. Solvents are generally classified by the polarity, and considered either polar or non. Because. What Is The Solvent Polarity.
From www.slideserve.com
PPT Solvent Effects PowerPoint Presentation ID3080477 What Is The Solvent Polarity the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. 23 rows solvent polarity. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. Because of the shape of the. This creates a slight imbalance of the. in this explainer, we will learn how to describe polar. What Is The Solvent Polarity.
From worksheetsabrale1b.z13.web.core.windows.net
Water As A Universal Solvent Notes What Is The Solvent Polarity the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. Solvents are generally classified by the polarity, and considered either polar or non. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. Solubility can be defined as the tendency. because water is polar, substances that are polar. What Is The Solvent Polarity.
From bceweb.org
Solvent Polarity Chart A Visual Reference of Charts Chart Master What Is The Solvent Polarity 23 rows solvent polarity. polar solvents are best for dissolving polar reactants (such as ions); This creates a slight imbalance of the. Solubility can be defined as the tendency. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. today, we will explore how polar. What Is The Solvent Polarity.
From mavink.com
Solvent Polarity Chart For Tlc What Is The Solvent Polarity Solvents are generally classified by the polarity, and considered either polar or non. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Because of the shape of the. This. What Is The Solvent Polarity.
From www.researchgate.net
Schematic diagram of the effect of solvent polarity on final structure What Is The Solvent Polarity polar solvents are best for dissolving polar reactants (such as ions); Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. 23 rows solvent polarity. today, we will explore how polar organic solvents are, and will test what mixes with what. the two hydrogen atoms are not on opposite sides of the oxygen,. What Is The Solvent Polarity.
From sciencenotes.org
Why Is Water Called the Universal Solvent? What Is The Solvent Polarity Solubility can be defined as the tendency. 23 rows solvent polarity. This creates a slight imbalance of the. Solvents are generally classified by the polarity, and considered either polar or non. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. Because of the shape of the. solvent polarity refers to the ability of a. What Is The Solvent Polarity.
From www.chegg.com
Solved Determine the solvent polarity index for the What Is The Solvent Polarity today, we will explore how polar organic solvents are, and will test what mixes with what. Solubility can be defined as the tendency. 23 rows solvent polarity. because water is polar, substances that are polar or ionic will dissolve in it. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances,. What Is The Solvent Polarity.
From www.chemistrysteps.com
The Role of Solvent in SN1, SN2, E1 and E2 Reactions Chemistry Steps What Is The Solvent Polarity solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. in this explainer, we will learn how to describe polar and nonpolar solvents. This creates a slight imbalance of the. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an. What Is The Solvent Polarity.
From egpat.com
Solvatochromism The effect of polarity of solvent on lamba max What Is The Solvent Polarity the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. 23 rows solvent polarity. polar. What Is The Solvent Polarity.
From www.masterorganicchemistry.com
Polar Protic? Polar Aprotic? Nonpolar? All About Solvents What Is The Solvent Polarity Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. This creates a slight imbalance of the. Solvents are generally classified by the polarity, and considered either polar or non. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. 23 rows solvent polarity.. What Is The Solvent Polarity.
From www.slideserve.com
PPT Chapter 2 Chemistry PowerPoint Presentation, free What Is The Solvent Polarity Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. in this explainer, we will learn how to describe polar and nonpolar solvents. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. polar solvents are best for dissolving polar reactants (such as. What Is The Solvent Polarity.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3746599 What Is The Solvent Polarity solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Because of the shape of the. This creates a slight imbalance of the. Solvents are generally classified by the polarity, and considered either polar or non. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine. What Is The Solvent Polarity.
From www.researchgate.net
Solvent polarity and eluotropic strength (ɛ°) Download Scientific Diagram What Is The Solvent Polarity in this explainer, we will learn how to describe polar and nonpolar solvents. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. This creates a slight imbalance of the. Solubility can be defined as the tendency. today, we will explore how polar organic solvents are, and will test what. What Is The Solvent Polarity.
From www.researchgate.net
Solvent polarity, l max , and ringclosing rate for Ox 1M and Ox 2M What Is The Solvent Polarity the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. today, we will explore how polar organic solvents are, and will test what mixes with what. Because of the shape of the. solvent polarity refers to the. What Is The Solvent Polarity.
From mavink.com
Polarity Chart Of Solvents What Is The Solvent Polarity Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. Solvents are generally classified by the polarity, and considered either polar or non. polar solvents are best for dissolving polar reactants (such as ions); in this explainer, we will learn how to describe polar and nonpolar solvents. This creates a slight imbalance of the. . What Is The Solvent Polarity.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID269800 What Is The Solvent Polarity today, we will explore how polar organic solvents are, and will test what mixes with what. Because of the shape of the. 23 rows solvent polarity. Solubility can be defined as the tendency. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. polar solvents. What Is The Solvent Polarity.
From aceorganicchem.com
Organic Chem 15 For organic solvents, likes dissolve likes What Is The Solvent Polarity Solubility can be defined as the tendency. because water is polar, substances that are polar or ionic will dissolve in it. This creates a slight imbalance of the. Solvents are generally classified by the polarity, and considered either polar or non. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle.. What Is The Solvent Polarity.
From chempedia.info
Polarity index Solvent Big Chemical Encyclopedia What Is The Solvent Polarity 23 rows solvent polarity. Because of the shape of the. polar solvents are best for dissolving polar reactants (such as ions); in this explainer, we will learn how to describe polar and nonpolar solvents. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. today, we will explore. What Is The Solvent Polarity.
From mavink.com
Solvent Polarity Chart For Tlc What Is The Solvent Polarity Solvents are generally classified by the polarity, and considered either polar or non. in this explainer, we will learn how to describe polar and nonpolar solvents. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. This creates a slight imbalance of the. today, we will. What Is The Solvent Polarity.
From www.researchgate.net
1 Bulk solvent polarity parameters. Download Table What Is The Solvent Polarity polar solvents are best for dissolving polar reactants (such as ions); This creates a slight imbalance of the. in this explainer, we will learn how to describe polar and nonpolar solvents. because water is polar, substances that are polar or ionic will dissolve in it. 23 rows solvent polarity. solvent polarity refers to the ability. What Is The Solvent Polarity.
From alexmistry.z13.web.core.windows.net
Order Of Solvent Polarity What Is The Solvent Polarity in this explainer, we will learn how to describe polar and nonpolar solvents. Solvents are generally classified by the polarity, and considered either polar or non. 23 rows solvent polarity. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. today, we will explore how. What Is The Solvent Polarity.
From www.chegg.com
Solved Determine the solvent polarity index for each HPLC What Is The Solvent Polarity today, we will explore how polar organic solvents are, and will test what mixes with what. polar solvents are best for dissolving polar reactants (such as ions); This creates a slight imbalance of the. because water is polar, substances that are polar or ionic will dissolve in it. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. What Is The Solvent Polarity.
From mungfali.com
Solvent Polarity Chart What Is The Solvent Polarity polar solvents are best for dissolving polar reactants (such as ions); Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. 23 rows solvent polarity. Because of the shape of the. solvent polarity refers to the. What Is The Solvent Polarity.
From www.britannica.com
polarity Definition & Examples Britannica What Is The Solvent Polarity in this explainer, we will learn how to describe polar and nonpolar solvents. today, we will explore how polar organic solvents are, and will test what mixes with what. 23 rows solvent polarity. Solubility can be defined as the tendency. This creates a slight imbalance of the. solvent polarity refers to the ability of a solvent. What Is The Solvent Polarity.
From www.slideserve.com
PPT Unit 6 Chemical Bonding PowerPoint Presentation, free download What Is The Solvent Polarity This creates a slight imbalance of the. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Solubility can be defined as the tendency. polar solvents are best for dissolving polar reactants (such as. What Is The Solvent Polarity.
From www.aboutcleaningproducts.com
Solvent Science About Cleaning Products What Is The Solvent Polarity 23 rows solvent polarity. polar solvents are best for dissolving polar reactants (such as ions); Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. in this explainer, we will learn how to describe polar and nonpolar solvents. today, we will explore how polar organic solvents are, and will test what mixes with. What Is The Solvent Polarity.
From www.slideserve.com
PPT Alkyl halides PowerPoint Presentation, free download ID599053 What Is The Solvent Polarity Solubility can be defined as the tendency. 23 rows solvent polarity. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Because of the shape of the. because. What Is The Solvent Polarity.
From tutors.com
What is a solvent? Definition & Examples (Video) What Is The Solvent Polarity solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. Because of the shape of the. because water is polar, substances that are polar or ionic will dissolve in it. today, we will explore how polar organic solvents are, and will test what mixes with what.. What Is The Solvent Polarity.
From mungfali.com
Polarity Index Chart What Is The Solvent Polarity Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane diethylamine aniline. the two hydrogen atoms are not on opposite sides of the oxygen, but rather at an angle. Because of the shape of the. 23 rows solvent polarity. Solubility can be defined as the tendency. in this explainer, we will learn how to describe polar. What Is The Solvent Polarity.
From www.youtube.com
Solutions Lesson 3 Polarity YouTube What Is The Solvent Polarity solvent polarity refers to the ability of a solvent to dissolve polar or ionic substances, determined by its molecular structure and. polar solvents are best for dissolving polar reactants (such as ions); 23 rows solvent polarity. This creates a slight imbalance of the. Solvents are generally classified by the polarity, and considered either polar or non. . What Is The Solvent Polarity.