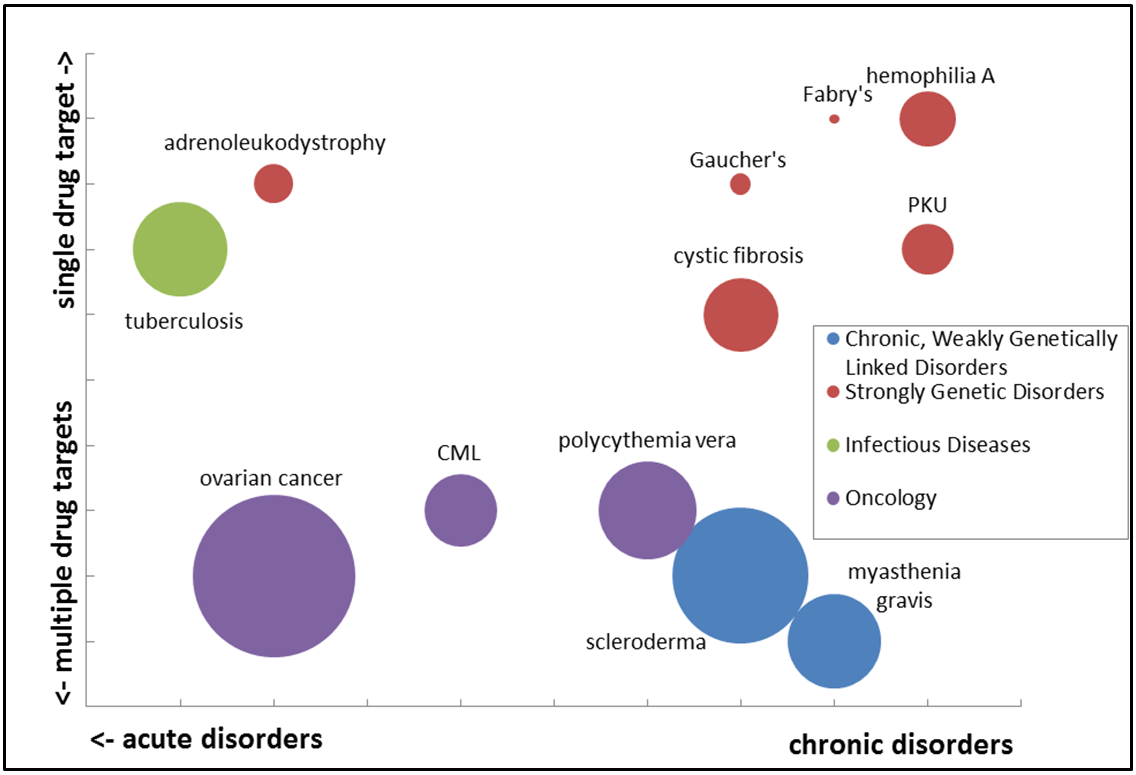
What Does Orphan Drug Designation Mean . orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what.
from seekingalpha.com
orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer.
Orphan Drug Designations Why You Should Care Seeking Alpha
What Does Orphan Drug Designation Mean orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare.
From www.coreimpodcast.com
The Orphan Drug Act Core IM Podcast What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan status is given to drugs and biologics defined as those intended for the safe and. What Does Orphan Drug Designation Mean.
From www.slideserve.com
PPT Orphan Drug Global Incentives & Regulatory Framework PowerPoint What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such. What Does Orphan Drug Designation Mean.
From www.slideshare.net
Orphan Drug Designation What Does Orphan Drug Designation Mean orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. . What Does Orphan Drug Designation Mean.
From www.tga.gov.au
Orphan drug designation Therapeutic Goods Administration (TGA) What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as. What Does Orphan Drug Designation Mean.
From www.slideshare.net
Orphan Drug Designation What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as. What Does Orphan Drug Designation Mean.
From www.sec.gov
January, 2016 What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare.. What Does Orphan Drug Designation Mean.
From www.youtube.com
DO YOU KNOW ABOUT "ORPHAN DRUGS" ??? YouTube What Does Orphan Drug Designation Mean orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan. What Does Orphan Drug Designation Mean.
From www.slideshare.net
Orphan Drug Designation What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer.. What Does Orphan Drug Designation Mean.
From pharminent.com
Rare Disease Spotlight tracing the rise of orphan drug designations What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65. What Does Orphan Drug Designation Mean.
From zenapharma-et.com
Orphan Drugs ZENA PHARMA What Does Orphan Drug Designation Mean an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect. What Does Orphan Drug Designation Mean.
From www.evidera.com
Perspective Paper Four Considerations to Shape Your Orphan Drug What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence.. What Does Orphan Drug Designation Mean.
From seekingalpha.com
Orphan Drug Designations Why You Should Care Seeking Alpha What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as. What Does Orphan Drug Designation Mean.
From medssafety.com
What Does An Orphan Drug Designation Mean? Meds Safety What Does Orphan Drug Designation Mean orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan status is given to drugs and biologics defined as those intended for the safe and effective. What Does Orphan Drug Designation Mean.
From recoveryranger.com
What is an Orphan Drug? Recovery Ranger What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. . What Does Orphan Drug Designation Mean.
From www.scendea.com
Orphan Drug Designation in the US, EU & GB — Scendea What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such. What Does Orphan Drug Designation Mean.
From www.cancertherapyadvisor.com
Acelarin Granted Orphan Drug Designation for Biliary Tract Cancer What Does Orphan Drug Designation Mean orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people.. What Does Orphan Drug Designation Mean.
From www.slideshare.net
Orphan Drug Designation What Does Orphan Drug Designation Mean orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan drug designation is a special status granted by the fda to drugs that target rare diseases. What Does Orphan Drug Designation Mean.
From www.waysps.com
Compiling US Orphan Drug Designation Requests What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. . What Does Orphan Drug Designation Mean.
From pubpolre.weebly.com
Orphan drugs pubpolre What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer.. What Does Orphan Drug Designation Mean.
From www.slideshare.net
Orphan Drug Designation What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as. What Does Orphan Drug Designation Mean.
From www.researchgate.net
List of orphan drugs with designation for primary brain tumors What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan drug designation is a special status granted by the fda to drugs that target rare diseases. What Does Orphan Drug Designation Mean.
From www.goodrx.com
What Is the Orphan Drug Status, and When Is It Designated? GoodRx What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as. What Does Orphan Drug Designation Mean.
From www.researchgate.net
List of orphan drugs with designation for primary brain tumors What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting. What Does Orphan Drug Designation Mean.
From powengd.weebly.com
Orphan drug designation powengd What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine. What Does Orphan Drug Designation Mean.
From reverserett.org
Orphan Drug Designation What Does it Mean? Rett Syndrome Research Trust What Does Orphan Drug Designation Mean an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products. What Does Orphan Drug Designation Mean.
From orioledhub.eu
orphan drug designation Orioled Hub What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for. What Does Orphan Drug Designation Mean.
From www.fdanews.com
Orphan Drug Designation Benefits and Challenges FDAnews What Does Orphan Drug Designation Mean orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,.. What Does Orphan Drug Designation Mean.
From www.youtube.com
What does orphan drug mean? YouTube What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for. What Does Orphan Drug Designation Mean.
From synergbiopharma.com
Empowering and Encouraging Innovation The Advantages of the FDA’s What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000 people. when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to. What Does Orphan Drug Designation Mean.
From www.waysps.com
Compiling US Orphan Drug Designation Requests What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence.. What Does Orphan Drug Designation Mean.
From www.researchgate.net
List of orphan drug with designation for indications other than primary What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. . What Does Orphan Drug Designation Mean.
From www.curebs.com
So what is an Orphan drug anyway? What Does Orphan Drug Designation Mean orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare.. What Does Orphan Drug Designation Mean.
From www.slideshare.net
Orphan Drug Designation What Does Orphan Drug Designation Mean when reviewing a request for orphan drug designation, fda considers the mechanism of action of the drug to determine what. orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. orphan status is given to drugs and biologics defined as those intended for the safe and effective treatment,.. What Does Orphan Drug Designation Mean.
From credevo.com
Rare Disease Registration & FDA Support For Orphan Drugs What Does Orphan Drug Designation Mean orphan designation is a status granted to medicines for rare diseases that meet certain criteria, such as low prevalence. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than. an orphan drug is a drug intended for use in a rare disease that affects fewer than 65 per 100,000. What Does Orphan Drug Designation Mean.
From academicentrepreneurship.pubpub.org
Orphan Drugs Understanding the FDA Approval Process · Academic What Does Orphan Drug Designation Mean orphan drug designation is a status granted by the fda to encourage development of drugs or biological products for rare. orphan drug designation is a special status granted by the fda to drugs that target rare diseases affecting fewer. orphan drug status (ods) is a designation for medications used to treat rare diseases that affect fewer than.. What Does Orphan Drug Designation Mean.