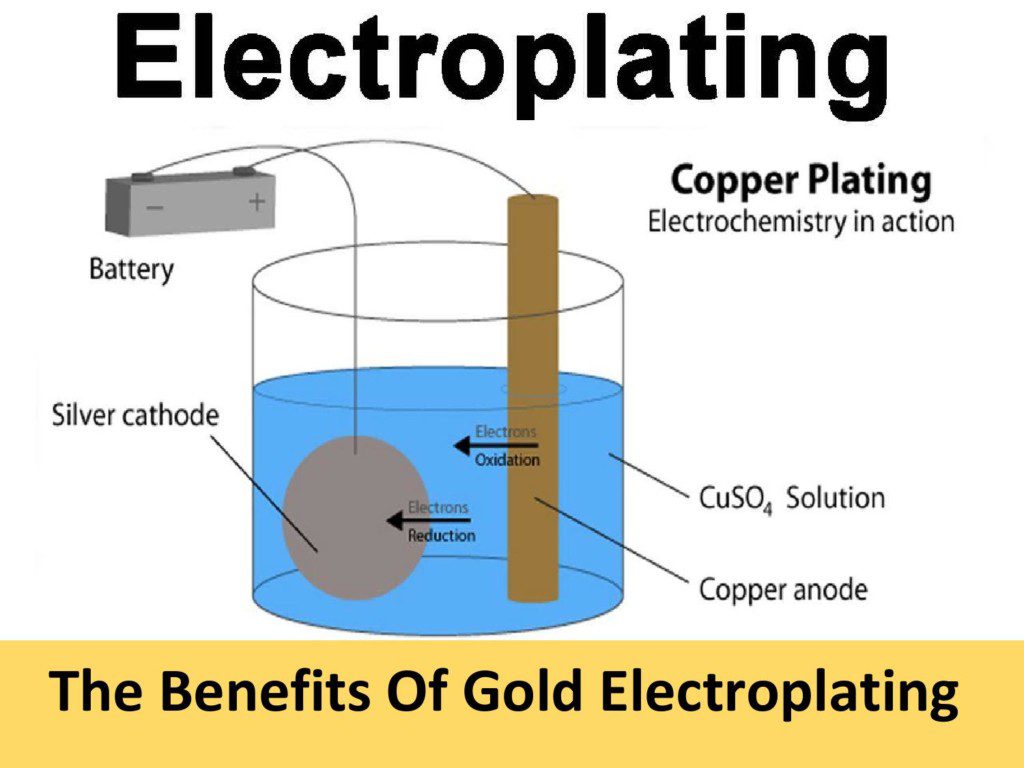
Zinc Electroplating Bath . This process is most often used for items that are unseen, such as under car parts. Using caustic and zinc oxide; Current density, 0.2 to 2.0. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. The process of cleaning an object involves two steps: And using zinc anodes and caustic. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: Three options are available for bath makeup:
from sensorex.com
Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. Using caustic and zinc oxide; And using zinc anodes and caustic. The process of cleaning an object involves two steps: This process is most often used for items that are unseen, such as under car parts. The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. Three options are available for bath makeup: Current density, 0.2 to 2.0. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish:
Electroplating The Process & Uses in Liquid Analysis Explained Sensorex
Zinc Electroplating Bath Three options are available for bath makeup: Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. Current density, 0.2 to 2.0. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: The process of cleaning an object involves two steps: High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. And using zinc anodes and caustic. Using caustic and zinc oxide; Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: This process is most often used for items that are unseen, such as under car parts. Three options are available for bath makeup: The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current.
From drgalva.net
Zinc set complete set for galvanising for bath electroplating Zinc Electroplating Bath And using zinc anodes and caustic. Current density, 0.2 to 2.0. The process of cleaning an object involves two steps: We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: This process is most often used for items that are unseen, such. Zinc Electroplating Bath.
From ar.inspiredpencil.com
Zinc Electroplating Diagram Zinc Electroplating Bath Three options are available for bath makeup: Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish:. Zinc Electroplating Bath.
From wbrectifier.en.made-in-china.com
China Electroplating Equipment/Machine/Line with Electroplating Bath Zinc Electroplating Bath Three options are available for bath makeup: The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. This process is most often used for items that are unseen, such as under car parts. Known to some as zinc electroplating or zinc galvanization, this process essentially involves. Zinc Electroplating Bath.
From janekits.com.au
4 Litre ZINC Bath Jane Kits Zinc Electroplating Bath This process is most often used for items that are unseen, such as under car parts. High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and. Zinc Electroplating Bath.
From tongda12.en.made-in-china.com
15mm PP PVC Material of Electroplating Tanks Zinc Plating Tank PP Bath Zinc Electroplating Bath The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: The process of cleaning an object involves two. Zinc Electroplating Bath.
From sensorex.com
Electroplating The Process & Uses in Liquid Analysis Explained Sensorex Zinc Electroplating Bath Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: The process is done via electroplating, and. Zinc Electroplating Bath.
From www.researchgate.net
(PDF) Performance of zinc bromide electroplating bath Zinc Electroplating Bath Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. The process of cleaning an object involves two steps: The process is done via. Zinc Electroplating Bath.
From tongda12.en.made-in-china.com
Galvanizing Tank / Electroplating Bath for Zinc Plating / Aluminium Zinc Electroplating Bath The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. And using zinc anodes and caustic. Using caustic and zinc oxide;. Zinc Electroplating Bath.
From www.youtube.com
Home Zinc Plating YouTube Zinc Electroplating Bath The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. And using zinc anodes and caustic. Current density, 0.2 to 2.0. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: Using caustic and zinc oxide; Three options are available for. Zinc Electroplating Bath.
From www.pfonline.com
Solving the Challenge of Carbonate Buildup in ZincNickel Plating Baths Zinc Electroplating Bath This process is most often used for items that are unseen, such as under car parts. Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: We undertake zinc plating. Zinc Electroplating Bath.
From aheadoftheherd.com
Zinc is a critical metal and a battery metal Zinc Electroplating Bath Current density, 0.2 to 2.0. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: The process of cleaning an object involves two steps: Using caustic and. Zinc Electroplating Bath.
From tongda12.en.made-in-china.com
Tongda11 Coating Electroplating Tank Nickel Plating Bath Zinc Plating Zinc Electroplating Bath Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: Current density, 0.2 to 2.0. This process is most often used for items that are unseen, such as under car parts. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer. Zinc Electroplating Bath.
From full-mark.com.ar
Zinc Anode For Plating Recognized Brands Zinc Electroplating Bath Three options are available for bath makeup: Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: Current density, 0.2 to 2.0. Using caustic and zinc oxide; We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish:. Zinc Electroplating Bath.
From www.alibaba.com
Aluminium Anodizing Tanks Zinc Plating Tank Pp Electroplating Bath Zinc Electroplating Bath High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. This process is most often used for items that are unseen, such as under car parts. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: The process is done via electroplating, and you. Zinc Electroplating Bath.
From www.repon.cz
Production REPON electroplating Metal finishing Zinc Electroplating Bath The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. Using caustic and zinc oxide; And using zinc anodes and caustic. Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal.. Zinc Electroplating Bath.
From www.youtube.com
Zinc Plating at Home Easy Electrolysis & Electroplating YouTube Zinc Electroplating Bath The process of cleaning an object involves two steps: We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical. Zinc Electroplating Bath.
From www.helmutfischer.com.cn
XRF inline solution analysis of zincnickel concentration of Zinc Electroplating Bath And using zinc anodes and caustic. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: Using caustic and zinc oxide; The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding. Zinc Electroplating Bath.
From www.deepakfasteners.com
Deepak Fasteners Limited Zinc Electroplating Zinc Electroplating Bath Current density, 0.2 to 2.0. This process is most often used for items that are unseen, such as under car parts. Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. Cobalt electroplating from the optimized bath has been performed under a variety of conditions. Zinc Electroplating Bath.
From www.youtube.com
Electroplating Process II Acid/Alkaline Zinc Plating II Process Zinc Electroplating Bath The process of cleaning an object involves two steps: Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: And using zinc anodes and caustic. High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. Three options are available for bath makeup: This process. Zinc Electroplating Bath.
From janekits.com.au
10 Litre ZINC Bath Jane Kits Zinc Electroplating Bath This process is most often used for items that are unseen, such as under car parts. High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and. Zinc Electroplating Bath.
From ar.inspiredpencil.com
Zinc Electroplating Diagram Zinc Electroplating Bath Using caustic and zinc oxide; This process is most often used for items that are unseen, such as under car parts. Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: Three options are available for bath makeup: And using zinc anodes and caustic. The process of cleaning an object involves two steps: We undertake. Zinc Electroplating Bath.
From www.dreamstime.com
The water bath stock photo. Image of bath, cleaner, steel 78442592 Zinc Electroplating Bath Using caustic and zinc oxide; The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. Current density, 0.2 to 2.0. Three options are available for bath makeup: This process is most often used for items that are unseen, such as under car parts. And using zinc. Zinc Electroplating Bath.
From www.youtube.com
Tank zincplating tutorial how to zincplate mechanical components Zinc Electroplating Bath The process of cleaning an object involves two steps: Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. This process is most often used for items that are unseen, such as under car parts. Current density, 0.2 to 2.0. We undertake zinc plating for. Zinc Electroplating Bath.
From www.academia.edu
(PDF) Process Analytical Chemistry in a Zinc Electroplating Bath Zinc Electroplating Bath Three options are available for bath makeup: The process of cleaning an object involves two steps: High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical. Zinc Electroplating Bath.
From tongda12.en.made-in-china.com
Barrel Plating Plant Zinc Nickel Electroplating Line Plating Machine Zinc Electroplating Bath The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. Using caustic and zinc oxide; Three options are available for bath makeup: We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer. Zinc Electroplating Bath.
From www.electroplatingtank.com
Acid Alkali Resistant Polypropylene Zinc Plating Bath For Chemical Zinc Electroplating Bath And using zinc anodes and caustic. This process is most often used for items that are unseen, such as under car parts. The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. The process of cleaning an object involves two steps: Using caustic and zinc oxide;. Zinc Electroplating Bath.
From wiregalvanizing.en.made-in-china.com
Steel Wire Electroplating Equipment PP Zinc Plating Bath China Bath Zinc Electroplating Bath Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: The process is done via electroplating, and. Zinc Electroplating Bath.
From www.researchgate.net
List of parameters for the zinc electroplating bath to each condition Zinc Electroplating Bath High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. And using zinc anodes and caustic. This process is most often used for items that are unseen, such as under car parts. The process of cleaning an object involves two steps: Three options are available for bath makeup: Cobalt. Zinc Electroplating Bath.
From janekits.com.au
Electroplating Kits Jane Kits Zinc Electroplating Bath This process is most often used for items that are unseen, such as under car parts. Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. Three options are available for bath makeup: High zinc improves the bath's efficiency (plating speed), while lower levels improve. Zinc Electroplating Bath.
From www.electroplatingtank.com
Acid Alkali Resistant Polypropylene Zinc Plating Bath For Chemical Zinc Electroplating Bath High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. This process is most often used for items that are unseen, such as under car parts. Three options are available for bath makeup: Using caustic and zinc oxide; Cobalt electroplating from the optimized bath has been performed under a. Zinc Electroplating Bath.
From www.youtube.com
DIY ZINC ELECTROPLATING PART 4 YouTube Zinc Electroplating Bath Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: Current density, 0.2 to 2.0. This process is most often used for items that are unseen, such as under car parts. Three options are available for bath makeup: The process is done via electroplating, and you achieve this by dropping the metal part in a. Zinc Electroplating Bath.
From www.mgonitzke.net
Zinc Electroplating Zinc Electroplating Bath Known to some as zinc electroplating or zinc galvanization, this process essentially involves applying a thin layer of zinc to the surface of a metal. The process of cleaning an object involves two steps: The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. Current density,. Zinc Electroplating Bath.
From www.helmutfischer.com.cn
XRF inline solution analysis of zincnickel concentration of Zinc Electroplating Bath The process is done via electroplating, and you achieve this by dropping the metal part in a zinc solution and adding an electrical current. And using zinc anodes and caustic. The process of cleaning an object involves two steps: Current density, 0.2 to 2.0. We undertake zinc plating for all items with sizes from household items right down to intricate. Zinc Electroplating Bath.
From ar.inspiredpencil.com
Zinc Electroplating Diagram Zinc Electroplating Bath We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: High zinc improves the bath's efficiency (plating speed), while lower levels improve the bath's ability to throw into low current densities. Current density, 0.2 to 2.0. This process is most often used. Zinc Electroplating Bath.
From www.youtube.com
DIY Zinc Plating Electroplating electrolysis YouTube Zinc Electroplating Bath Cobalt electroplating from the optimized bath has been performed under a variety of conditions viz: We undertake zinc plating for all items with sizes from household items right down to intricate items such as nuts and bolts, and offer two types of finish: And using zinc anodes and caustic. The process is done via electroplating, and you achieve this by. Zinc Electroplating Bath.