Bromide Ion Oxidation Reaction . Therefore, the ao of bromophenol. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Ferrate is shown to oxidize bromide forming bromate under certain conditions. Because the full reaction here is the combination of a. The bromide ions are being oxidized to form bromine gas. Hydrogen peroxide, ferric particles, and solutes. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular.
from www.slideshare.net
In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. The bromide ions are being oxidized to form bromine gas. Therefore, the ao of bromophenol. Because the full reaction here is the combination of a. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Ferrate is shown to oxidize bromide forming bromate under certain conditions. Hydrogen peroxide, ferric particles, and solutes. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds.
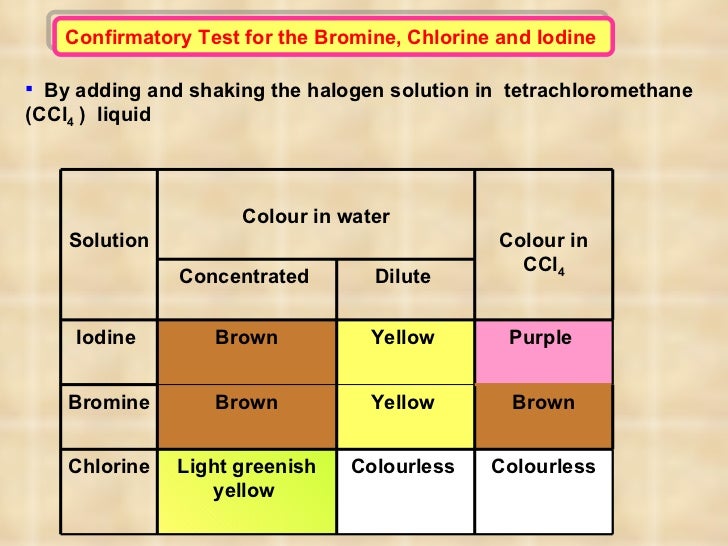
Oxidation & reduction
Bromide Ion Oxidation Reaction Because the full reaction here is the combination of a. Because the full reaction here is the combination of a. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Hydrogen peroxide, ferric particles, and solutes. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Ferrate is shown to oxidize bromide forming bromate under certain conditions. The bromide ions are being oxidized to form bromine gas. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Therefore, the ao of bromophenol.
From www.numerade.com
SOLVED Balance the following skeletal equations by using oxidation and Bromide Ion Oxidation Reaction However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources. Bromide Ion Oxidation Reaction.
From chemistrysteps.com
Reactions of Thiols Chemistry Steps Bromide Ion Oxidation Reaction Ferrate is shown to oxidize bromide forming bromate under certain conditions. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Because the full reaction here is the combination of a. However, no attempts have been published concerning the fate and role of bromide (br. Bromide Ion Oxidation Reaction.
From brainly.com
Consider the reaction of an alkyl bromide and hydroxide ion. OH Draw Bromide Ion Oxidation Reaction Ferrate is shown to oxidize bromide forming bromate under certain conditions. The bromide ions are being oxidized to form bromine gas. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Hydrogen peroxide, ferric particles, and solutes. In this review, an overview is given of. Bromide Ion Oxidation Reaction.
From www.slideshare.net
Oxidation & reduction Bromide Ion Oxidation Reaction Therefore, the ao of bromophenol. The bromide ions are being oxidized to form bromine gas. Ferrate is shown to oxidize bromide forming bromate under certain conditions. Hydrogen peroxide, ferric particles, and solutes. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. However, no attempts have been published concerning the fate and role of. Bromide Ion Oxidation Reaction.
From www.numerade.com
SOLVED ' Oxidation Reduction (Redox) reactions can be balanced using Bromide Ion Oxidation Reaction In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Because the full reaction here is the combination of a. Ferrate is shown to oxidize bromide forming bromate under certain conditions. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of. Bromide Ion Oxidation Reaction.
From joieqcwyg.blob.core.windows.net
Bromine Plus Ion at David Martinelli blog Bromide Ion Oxidation Reaction The bromide ions are being oxidized to form bromine gas. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Therefore, the ao of bromophenol. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic. Bromide Ion Oxidation Reaction.
From solvedlib.com
Calculate E°cell for the oxidation of bromine ions… SolvedLib Bromide Ion Oxidation Reaction In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Because the full reaction here is the combination of a. Ferrate is shown to oxidize bromide forming bromate under certain conditions. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for. Bromide Ion Oxidation Reaction.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记2.3.4 Halide Ion Reactions翰林国际教育 Bromide Ion Oxidation Reaction Hydrogen peroxide, ferric particles, and solutes. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Because the full reaction here is the combination of a. The bromide ions. Bromide Ion Oxidation Reaction.
From www.youtube.com
Reaction of Sodium (Na) with Bromine (Br2) YouTube Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Because the full reaction here is the combination of a. Therefore, the ao of bromophenol. Ferrate is shown to oxidize bromide forming bromate under certain conditions. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during. Bromide Ion Oxidation Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation Bromide Ion Oxidation Reaction Hydrogen peroxide, ferric particles, and solutes. Because the full reaction here is the combination of a. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. The bromide ions. Bromide Ion Oxidation Reaction.
From openpress.usask.ca
12.7. Oxidation of Alcohols via Elimination Introduction to Organic Bromide Ion Oxidation Reaction However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. The bromide ions are being oxidized to form bromine gas. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Ferrate is shown to oxidize bromide forming bromate under. Bromide Ion Oxidation Reaction.
From www.toppr.com
The stoichiometric equation for the oxidation of bromide ions by Bromide Ion Oxidation Reaction However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Therefore, the ao of bromophenol. Ferrate is shown to oxidize bromide forming bromate under certain conditions. Hydrogen peroxide, ferric particles, and solutes. Because the full reaction here is the combination of a. Kinetics and mechanisms. Bromide Ion Oxidation Reaction.
From www.youtube.com
Quick video Balancing an oxidation reduction reaction in base [bromine Bromide Ion Oxidation Reaction In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. The bromide ions are being oxidized to form bromine gas. Therefore, the ao of bromophenol. Ferrate is shown to oxidize bromide forming bromate under certain conditions. Kinetics and mechanisms of aqueous ozone reactions with bromide,. Bromide Ion Oxidation Reaction.
From pubs.acs.org
and Mechanism of Bromate−Bromide Reaction Catalyzed by Acetate Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Therefore, the ao of bromophenol. Hydrogen peroxide, ferric particles, and solutes. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. The bromide ions are being oxidized to form. Bromide Ion Oxidation Reaction.
From www.slideserve.com
PPT Electrophilic Aromatic Substitution (Bromination of Toluene Bromide Ion Oxidation Reaction Therefore, the ao of bromophenol. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Hydrogen peroxide, ferric particles, and solutes. Kinetics and mechanisms of aqueous ozone reactions with. Bromide Ion Oxidation Reaction.
From www.mdpi.com
Molecules Free FullText Tetrabutylammonium Bromide (TBAB Bromide Ion Oxidation Reaction Therefore, the ao of bromophenol. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for. Bromide Ion Oxidation Reaction.
From loehniqha.blob.core.windows.net
What Is The Valency Of Bromide at Manuel Chappelle blog Bromide Ion Oxidation Reaction Therefore, the ao of bromophenol. The bromide ions are being oxidized to form bromine gas. Ferrate is shown to oxidize bromide forming bromate under certain conditions. Hydrogen peroxide, ferric particles, and solutes. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo. Bromide Ion Oxidation Reaction.
From www.youtube.com
Redox balance Br2 = BrO3 + Br Ion electron method Half reaction Bromide Ion Oxidation Reaction The bromide ions are being oxidized to form bromine gas. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Kinetics and mechanisms of aqueous ozone reactions with bromide,. Bromide Ion Oxidation Reaction.
From passmyexams.co.uk
Oxidation of Ethanol Easy exam revision notes for GSCE Chemistry Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Hydrogen peroxide, ferric particles, and solutes. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative. Bromide Ion Oxidation Reaction.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Bromide Ion Oxidation Reaction Hydrogen peroxide, ferric particles, and solutes. Therefore, the ao of bromophenol. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic. Bromide Ion Oxidation Reaction.
From www.numerade.com
SOLVED Write equations for the halfreactions that occur in the Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Therefore, the ao of bromophenol. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex. Bromide Ion Oxidation Reaction.
From spmchemistry.blog.onlinetuition.com.my
Examples of Redox Reaction Displacement of Halogen SPM Chemistry Bromide Ion Oxidation Reaction However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Because the full reaction here is the combination of a. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular.. Bromide Ion Oxidation Reaction.
From chem.libretexts.org
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts Bromide Ion Oxidation Reaction Therefore, the ao of bromophenol. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Because the full reaction here is the combination of a. The bromide ions are being oxidized to form bromine gas. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite,. Bromide Ion Oxidation Reaction.
From www.toppr.com
The stoichiometric equation for the oxidation of bromide ions by Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. The bromide ions are being oxidized to form bromine gas. Hydrogen peroxide, ferric particles, and solutes. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Because the full reaction here is the combination of a. In this. Bromide Ion Oxidation Reaction.
From www.chegg.com
Solved The oxidation of bromide ion by persulfate is given Bromide Ion Oxidation Reaction Hydrogen peroxide, ferric particles, and solutes. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Because the full reaction here is the combination of a. The bromide ions. Bromide Ion Oxidation Reaction.
From www.chegg.com
Solved The reaction between bromate ions and bromide ions in Bromide Ion Oxidation Reaction In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. The bromide ions. Bromide Ion Oxidation Reaction.
From www.chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Bromide Ion Oxidation Reaction In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Hydrogen peroxide, ferric particles, and solutes. Therefore, the ao of bromophenol. The bromide ions are being oxidized to form. Bromide Ion Oxidation Reaction.
From www.chemistrysteps.com
Oxidation of Monosaccharide Carbohydrates Chemistry Steps Bromide Ion Oxidation Reaction The bromide ions are being oxidized to form bromine gas. Because the full reaction here is the combination of a. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Therefore, the ao of bromophenol. In the aqueous bulk, oxidation of bromide by ozone involves. Bromide Ion Oxidation Reaction.
From www.numerade.com
SOLVEDbromide can be used to illustrate (b) The conversion of iron(Il Bromide Ion Oxidation Reaction Therefore, the ao of bromophenol. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. The bromide ions are being oxidized to form bromine gas. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In this review, an. Bromide Ion Oxidation Reaction.
From warreninstitute.org
Mastering Halohydrin Formation And Epoxidation Key Reactions For Alkenes Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Ferrate is shown to oxidize bromide forming bromate under certain conditions. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Hydrogen peroxide, ferric particles, and solutes. In the. Bromide Ion Oxidation Reaction.
From www.chegg.com
Solved Permanganate (MnO4 ') is a strong oxidizing agent of Bromide Ion Oxidation Reaction In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. In this review, an overview is given of chemical oxidants for the radical/electrophilic brominations starting from harmless bromide sources as an alternative for toxic molecular. Therefore, the ao of bromophenol. However, no attempts have been published concerning the fate and role of bromide (br. Bromide Ion Oxidation Reaction.
From www.mdpi.com
Molecules Free FullText DessMartin PeriodinaneMediated Oxidative Bromide Ion Oxidation Reaction Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. Therefore, the ao of bromophenol. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Ferrate is shown to oxidize bromide forming bromate under certain conditions. In this review,. Bromide Ion Oxidation Reaction.
From www.chegg.com
Solved Consider The Following Mechanism For The Oxidation... Bromide Ion Oxidation Reaction Because the full reaction here is the combination of a. Ferrate is shown to oxidize bromide forming bromate under certain conditions. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and. Bromide Ion Oxidation Reaction.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromide Ion Oxidation Reaction Ferrate is shown to oxidize bromide forming bromate under certain conditions. Hydrogen peroxide, ferric particles, and solutes. In the aqueous bulk, oxidation of bromide by ozone involves a [br•ooo −] complex as intermediate. Therefore, the ao of bromophenol. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao). Bromide Ion Oxidation Reaction.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction between Bromide Ion Oxidation Reaction The bromide ions are being oxidized to form bromine gas. Hydrogen peroxide, ferric particles, and solutes. Kinetics and mechanisms of aqueous ozone reactions with bromide, sulfite, hydrogen sulfite, iodide, and nitrite ions. However, no attempts have been published concerning the fate and role of bromide (br ‒) ions released during the anodic oxidation (ao) of organobromine compounds. Ferrate is shown. Bromide Ion Oxidation Reaction.