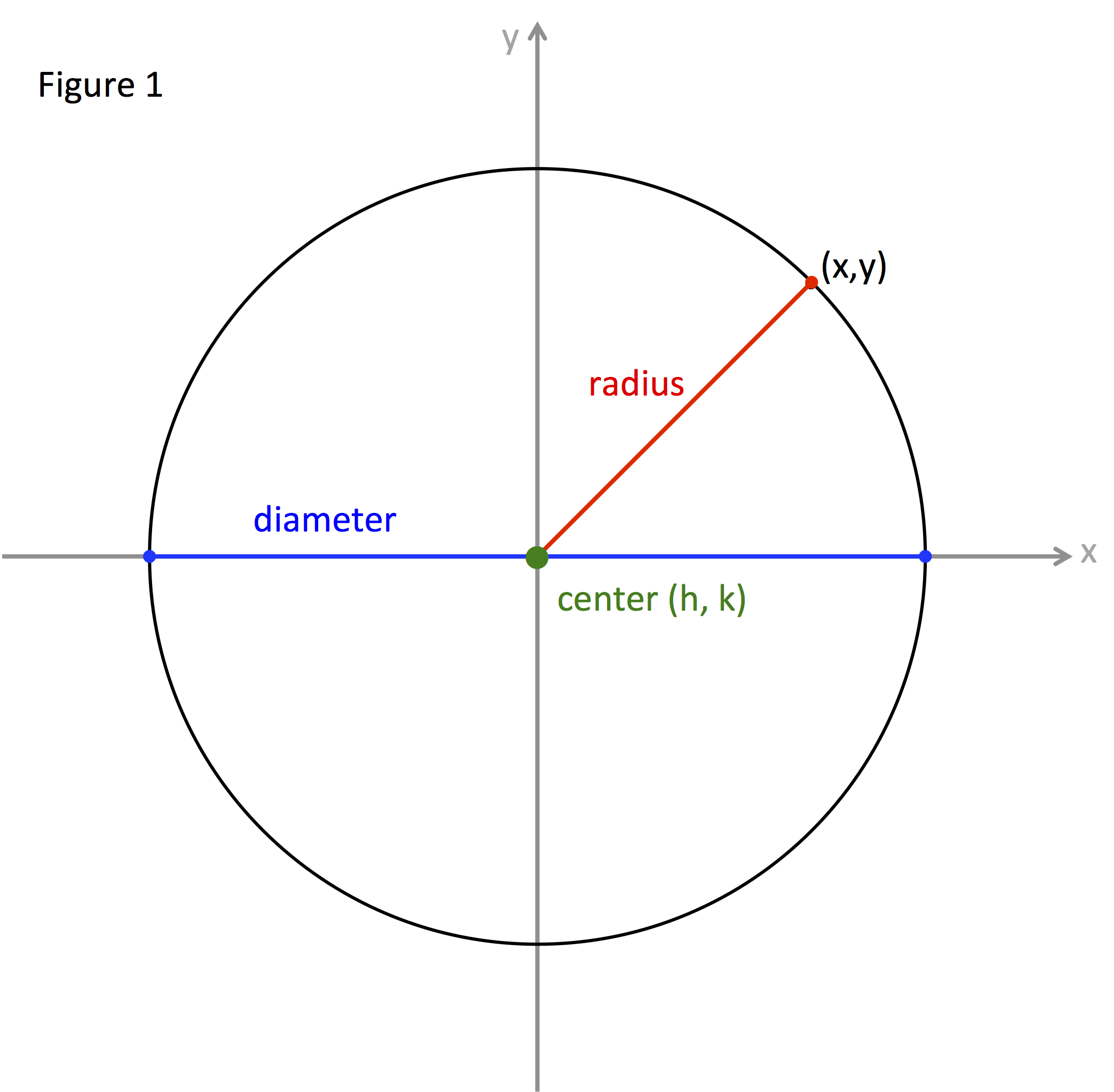
Crystal Radius Definition . The empirical crystal radii were. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. So what is crystal radius? Determining atomic radius from density, molar mass and crystal structure. I am aware that ionic radius is the radius of an atom's ion. The atoms are joined by. It is half the distance between the nuclei of two metal atoms in a metallic structure. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. I have found two different uses.
from truelfile902.weebly.com
The atoms are joined by. I am aware that ionic radius is the radius of an atom's ion. I have found two different uses. So what is crystal radius? Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. It is half the distance between the nuclei of two metal atoms in a metallic structure. The empirical crystal radii were. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively.
Blog Posts truelfile
Crystal Radius Definition The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. The atoms are joined by. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. So what is crystal radius? Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. I have found two different uses. Determining atomic radius from density, molar mass and crystal structure. It is half the distance between the nuclei of two metal atoms in a metallic structure. I am aware that ionic radius is the radius of an atom's ion. The empirical crystal radii were.
From www.sodialed.com
scrub radius definition RC Car Glossary Crystal Radius Definition The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. It is half the distance between the nuclei of two metal atoms in a metallic structure. I have found two different uses. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Determining atomic. Crystal Radius Definition.
From www.cuemath.com
Radius Definition, Formula & Example Cuemath Crystal Radius Definition The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. I have found two. Crystal Radius Definition.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.1.4 Atomic & Ionic Radius翰林国际教育 Crystal Radius Definition The atoms are joined by. I have found two different uses. So what is crystal radius? It is half the distance between the nuclei of two metal atoms in a metallic structure. I am aware that ionic radius is the radius of an atom's ion. The strategy is to use the following to calculate the volume of the unit cell,. Crystal Radius Definition.
From www.pinterest.com
Atomic Radius definition Half the distance between the nuclei of two Crystal Radius Definition The empirical crystal radii were. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. Determining atomic radius from density, molar mass and crystal structure. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. So what is. Crystal Radius Definition.
From kunduz.com
[ANSWERED] As per Pauling the radius of an a given crystal is i Crystal Radius Definition The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. It is half the distance between the nuclei of two metal atoms in a metallic structure. The empirical crystal radii were. So. Crystal Radius Definition.
From www.oxfordlearnersdictionaries.com
radius noun Definition, pictures, pronunciation and usage notes Crystal Radius Definition The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. It is half the distance between the nuclei of two metal atoms in a metallic structure. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. I have found two. Crystal Radius Definition.
From www.slideserve.com
PPT Unit VII Crystal structure PowerPoint Presentation ID1426222 Crystal Radius Definition So what is crystal radius? The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. I am aware that ionic radius is the radius of an atom's ion. The empirical crystal radii were. The strategy is to use the following to calculate the volume. Crystal Radius Definition.
From sciencenotes.org
Circle Facts Area, Circumference, Diameter, Radius Crystal Radius Definition I am aware that ionic radius is the radius of an atom's ion. It is half the distance between the nuclei of two metal atoms in a metallic structure. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The empirical crystal radii were. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and. Crystal Radius Definition.
From ppt-online.org
Particle Size Analysis презентация онлайн Crystal Radius Definition It is half the distance between the nuclei of two metal atoms in a metallic structure. The atoms are joined by. The empirical crystal radii were. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. I am aware that. Crystal Radius Definition.
From cabinet.matttroy.net
Atomic Radius Periodic Table Definition Matttroy Crystal Radius Definition The empirical crystal radii were. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. It is half the distance between the nuclei of two metal atoms in a metallic structure. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as. Crystal Radius Definition.
From 88guru.com
Atomic RadiusAn Overview 88Guru Crystal Radius Definition Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. It is half the distance between the nuclei of two metal atoms in a metallic structure. The atoms are joined by. The empirical crystal radii were. I am aware that ionic radius is the radius of an atom's ion. Table 1a.1 and 1a.2 list. Crystal Radius Definition.
From enthu.com
What is the Atomic Radius? EnthuZiastic Crystal Radius Definition So what is crystal radius? I have found two different uses. The empirical crystal radii were. It is half the distance between the nuclei of two metal atoms in a metallic structure. The atoms are joined by. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Determining. Crystal Radius Definition.
From dfwlokasin.weebly.com
Atomic radius definition dfwlokasin Crystal Radius Definition Determining atomic radius from density, molar mass and crystal structure. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. I am aware that ionic radius is the radius of an atom's ion. The atoms are joined by. The strategy. Crystal Radius Definition.
From truelfile902.weebly.com
Blog Posts truelfile Crystal Radius Definition So what is crystal radius? Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. I am aware that ionic radius is the radius of an atom's ion. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. It is half the distance between the nuclei of two metal atoms. Crystal Radius Definition.
From sciencenotes.org
Atomic Radius and Ionic Radius Crystal Radius Definition The atoms are joined by. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. I have found two different uses. So what is crystal radius? Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The half of inter nuclear distance between two adjacent atoms in a metallic crystal. Crystal Radius Definition.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Crystal Radius Definition So what is crystal radius? The empirical crystal radii were. The atoms are joined by. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. It is half the distance between the nuclei of two metal atoms in a metallic structure. The strategy is. Crystal Radius Definition.
From www.aiophotoz.com
Radius Of Circle Formula Examples Meaning Definition Images and Crystal Radius Definition I have found two different uses. It is half the distance between the nuclei of two metal atoms in a metallic structure. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. The atoms are joined by. The strategy is to use the following to calculate the volume of the unit cell, and then the length. Crystal Radius Definition.
From animalia-life.club
Atomic Radius Diagram Crystal Radius Definition I am aware that ionic radius is the radius of an atom's ion. It is half the distance between the nuclei of two metal atoms in a metallic structure. So what is crystal radius? The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the.. Crystal Radius Definition.
From www.geeksforgeeks.org
Radius of a Circle Definition, Formula How to Find Radius Crystal Radius Definition Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. I am aware that ionic radius is the radius of an atom's ion. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. The half of inter nuclear distance between two adjacent. Crystal Radius Definition.
From trends-periodic.weebly.com
Ionic Radii Periodic Trends Crystal Radius Definition The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. I have found two different uses. Determining atomic radius from density, molar mass and crystal structure. So what is crystal radius? The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as. Crystal Radius Definition.
From in.pinterest.com
Atomic radius Ionic radius, Chemistry education, Chemistry Crystal Radius Definition Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. Determining atomic radius from density, molar mass and crystal structure. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. I have found two different uses. The empirical. Crystal Radius Definition.
From www.youtube.com
Crystal Structures (Unit cell and Lattice parameters) YouTube Crystal Radius Definition The atoms are joined by. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. So what is crystal radius? I am aware that ionic radius is the radius of an atom's ion. The strategy is to use the following to calculate the volume. Crystal Radius Definition.
From ar.inspiredpencil.com
Periodic Table Ionic Radius Crystal Radius Definition It is half the distance between the nuclei of two metal atoms in a metallic structure. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The atoms are joined by. The half of inter nuclear distance between two adjacent. Crystal Radius Definition.
From www.pinterest.co.uk
Covalent Radius Definition and Trend in 2022 Covalent bonding, Radii Crystal Radius Definition The atoms are joined by. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. I am aware that ionic radius is the radius of an atom's ion. It is. Crystal Radius Definition.
From sciencenotes.org
Atomic Radius and Ionic Radius Crystal Radius Definition Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. I am aware that. Crystal Radius Definition.
From ar.inspiredpencil.com
Covalent Radius Crystal Radius Definition It is half the distance between the nuclei of two metal atoms in a metallic structure. I am aware that ionic radius is the radius of an atom's ion. So what is crystal radius? Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Determining atomic radius from density, molar mass and crystal structure. I have. Crystal Radius Definition.
From scientifictutor.org
Chem College Ionic Radius (Ionic Size) Scientific Tutor Crystal Radius Definition The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Determining atomic radius from density, molar mass and crystal structure. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and. Crystal Radius Definition.
From thechemistrynotes.com
Atomic radius Crystal Radius Definition Determining atomic radius from density, molar mass and crystal structure. It is half the distance between the nuclei of two metal atoms in a metallic structure. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. So what is crystal radius? I have found two different uses. The. Crystal Radius Definition.
From pressbooks.bccampus.ca
1.6 Periodic Variations in Element Properties Chemistry for Crystal Radius Definition So what is crystal radius? Determining atomic radius from density, molar mass and crystal structure. I am aware that ionic radius is the radius of an atom's ion. It is half the distance between the nuclei of two metal atoms in a metallic structure. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding.. Crystal Radius Definition.
From cabinet.matttroy.net
Atomic Radius Periodic Table Definition Matttroy Crystal Radius Definition Determining atomic radius from density, molar mass and crystal structure. The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. So what is crystal radius? Table 1a.1 and 1a.2 list pauling's empirical crystal radii and the univalent radii, respectively. Atoms in crystals are held together by electrostatic forces,. Crystal Radius Definition.
From ibrecap.com
Periodicity DP Chemistry IB Recap Crystal Radius Definition So what is crystal radius? I have found two different uses. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The atoms are joined by. The empirical crystal radii were. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or. Crystal Radius Definition.
From design.udlvirtual.edu.pe
What Is The Difference Between Radius And Diameter Of A Circle Design Crystal Radius Definition The strategy is to use the following to calculate the volume of the unit cell, and then the length of each side. Determining atomic radius from density, molar mass and crystal structure. The atoms are joined by. I have found two different uses. The empirical crystal radii were. The half of inter nuclear distance between two adjacent atoms in a. Crystal Radius Definition.
From www.media4math.com
DefinitionCircle ConceptsRadius of a Circle Media4Math Crystal Radius Definition I am aware that ionic radius is the radius of an atom's ion. I have found two different uses. The atoms are joined by. So what is crystal radius? The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. It is half the distance. Crystal Radius Definition.
From www.mathdoubts.com
Radius Crystal Radius Definition Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The atoms are joined by. So what is crystal radius? The empirical crystal radii were. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. Determining atomic radius. Crystal Radius Definition.
From wicati.com
Radius of Circle Formula, Definition Radius Formula (2023) Crystal Radius Definition The empirical crystal radii were. Atoms in crystals are held together by electrostatic forces, van der waals interactions, and covalent bonding. The atoms are joined by. The half of inter nuclear distance between two adjacent atoms in a metallic crystal is known as crystal radius or metallic radius or most of the. I have found two different uses. Table 1a.1. Crystal Radius Definition.