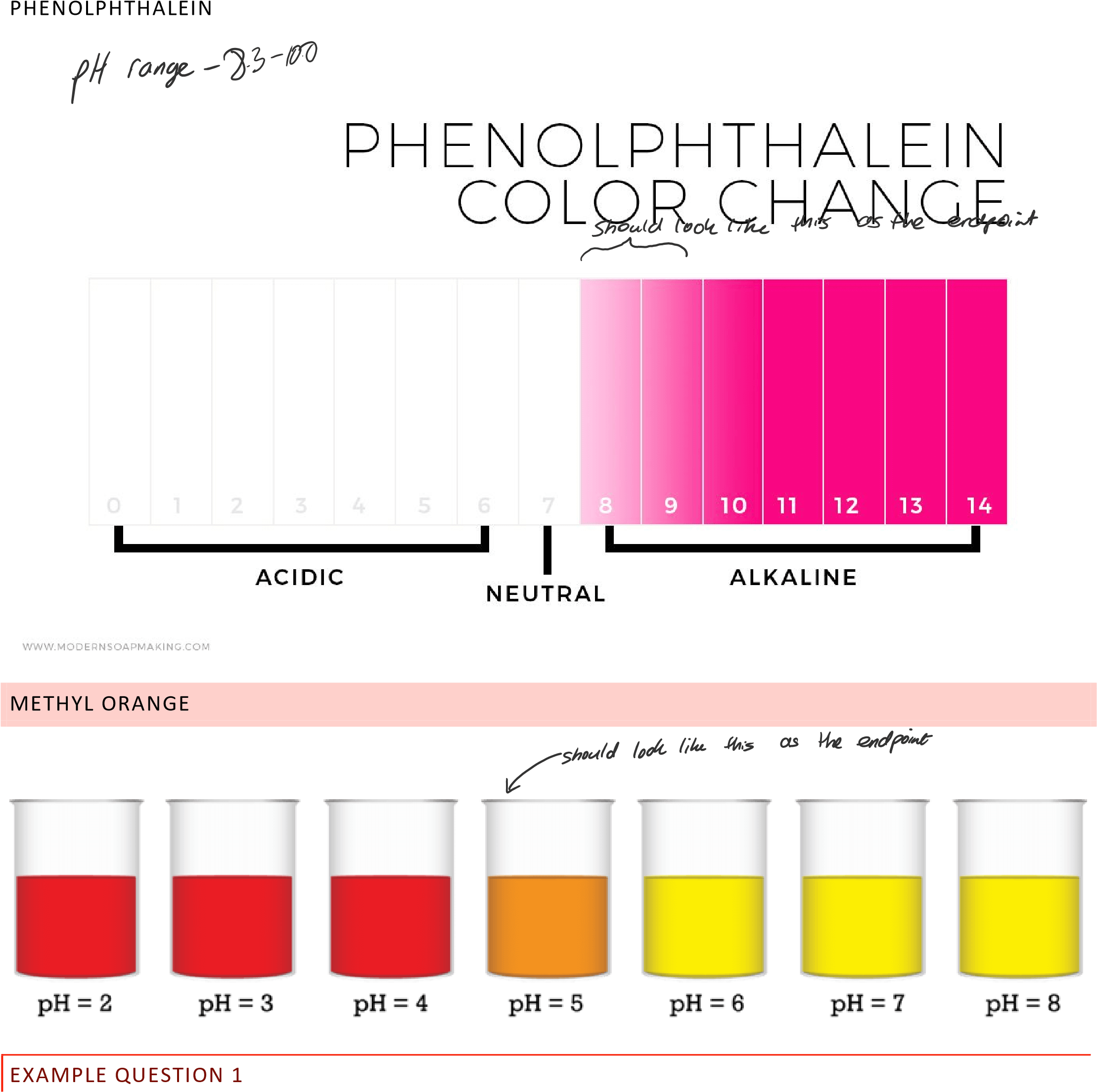
What Are Phenol Red And Phenolphthalein . In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Adding extra hydrogen ions shifts. In this case, the weak acid is colourless and its ion is bright pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. You titrate until the solution becomes colorless because that is as close to the. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.
from hsc.one
In this case, the weak acid is colourless and its ion is bright pink. Adding extra hydrogen ions shifts. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. You titrate until the solution becomes colorless because that is as close to the. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.
Module 6 Acids and Bases HSCOne
What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. You titrate until the solution becomes colorless because that is as close to the. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Adding extra hydrogen ions shifts.
From joelgordon.photoshelter.com
Chemisty experiement Phenolphthalein Joel Gordon Photography What Are Phenol Red And Phenolphthalein Substances such as phenolphthalein, which can be used to determine the ph of a solution,. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. You titrate until the solution becomes colorless because that is as close to the. Adding extra hydrogen ions. What Are Phenol Red And Phenolphthalein.
From www.researchgate.net
Phenol red absorbance spectra after mixing 11 with a pHadjusted What Are Phenol Red And Phenolphthalein You titrate until the solution becomes colorless because that is as close to the. In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. If you use phenolphthalein, you expect a. What Are Phenol Red And Phenolphthalein.
From thechemistrynotes.com
Phenol Classification, Preparation, Properties, Reactions, Uses What Are Phenol Red And Phenolphthalein For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. You titrate until the solution becomes colorless because that is as close to the. In this case, the weak acid is colourless and its ion is bright pink. Substances. What Are Phenol Red And Phenolphthalein.
From ar.inspiredpencil.com
Phenolphthalein What Are Phenol Red And Phenolphthalein In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. You titrate until the solution becomes colorless because that is as close to the. Phenolphthalein is another commonly. What Are Phenol Red And Phenolphthalein.
From thecontentauthority.com
Phenolphthalin vs Phenolphthalein Meaning And Differences What Are Phenol Red And Phenolphthalein In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
Phenols Phenolphthalein and Coupling Reaction YouTube What Are Phenol Red And Phenolphthalein If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Adding extra hydrogen ions shifts. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid.. What Are Phenol Red And Phenolphthalein.
From www.researchgate.net
The absorption spectrum of phenol at various illumination time What Are Phenol Red And Phenolphthalein Adding extra hydrogen ions shifts. In this case, the weak acid is colourless and its ion is bright pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. What Are Phenol Red And Phenolphthalein.
From www.watsons.ca
Phenolphthalein Indicator Watson's Barrels & Wine Making Supplies What Are Phenol Red And Phenolphthalein Adding extra hydrogen ions shifts. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. You titrate until the solution becomes colorless because that is as close to the. In this case, the weak acid is colourless and its ion is bright. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
Reaction of Phenol with Phthalic anhydride Phenolphthalein Organic What Are Phenol Red And Phenolphthalein In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this case, the weak acid is colourless and its ion is bright pink. Adding extra hydrogen ions shifts. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. For. What Are Phenol Red And Phenolphthalein.
From www.southernbiological.com
Phenol red Southern Biological What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. You titrate until the solution becomes colorless because that is as close to the. If you. What Are Phenol Red And Phenolphthalein.
From ar.inspiredpencil.com
Phenolphthalein What Are Phenol Red And Phenolphthalein For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Adding extra hydrogen ions shifts. In this case, the weak acid is colourless and its ion is bright pink. Substances such as phenolphthalein, which can be used to determine. What Are Phenol Red And Phenolphthalein.
From www.homesciencetools.com
Phenolphthalein Solution, 30 ml Home Science Tools What Are Phenol Red And Phenolphthalein If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. You titrate until the solution becomes colorless because that is as close to the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. For example,. What Are Phenol Red And Phenolphthalein.
From hsc.one
Module 6 Acids and Bases HSCOne What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Adding extra hydrogen ions shifts. In this case, the weak acid is colourless and its ion is bright pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. Substances such as phenolphthalein, which can be used to determine the. What Are Phenol Red And Phenolphthalein.
From stock.adobe.com
Color of pH indicator. The indicator color changes. Educational What Are Phenol Red And Phenolphthalein You titrate until the solution becomes colorless because that is as close to the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. Adding extra hydrogen ions. What Are Phenol Red And Phenolphthalein.
From www.britannica.com
Phenolphthalein pH indicator, acidbase titration, indicator dye What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. You titrate until the solution becomes colorless because that is as close to the. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. In more. What Are Phenol Red And Phenolphthalein.
From www.researchgate.net
Absorbance spectra of phenol red at different pH values. Download What Are Phenol Red And Phenolphthalein If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate. What Are Phenol Red And Phenolphthalein.
From mavink.com
Phenolphthalein Ph Range What Are Phenol Red And Phenolphthalein You titrate until the solution becomes colorless because that is as close to the. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. For. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
Preparation of Phenolphthalein Indicator solution YouTube What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. You titrate until the solution becomes colorless because that is as close to the. Adding extra hydrogen ions shifts. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6. What Are Phenol Red And Phenolphthalein.
From mungfali.com
Phenol Red Color Chart What Are Phenol Red And Phenolphthalein If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. You titrate until the solution. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
qualitative test for Phenols YouTube What Are Phenol Red And Phenolphthalein Adding extra hydrogen ions shifts. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. You titrate until the solution becomes colorless because that is as close to the. Substances such as phenolphthalein, which can be used to determine. What Are Phenol Red And Phenolphthalein.
From slideplayer.com
Phenol SubjectOC & UP( ) ppt download What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. You titrate until the solution becomes colorless because that is as close to the. In this case, the weak acid is colourless and its ion is bright pink. Substances such. What Are Phenol Red And Phenolphthalein.
From sielc.com
Phenol red SIELC What Are Phenol Red And Phenolphthalein Substances such as phenolphthalein, which can be used to determine the ph of a solution,. In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is another commonly used indicator. What Are Phenol Red And Phenolphthalein.
From dayaexir.com
خرید معرف فنل فتالئین (phenolphthalein) شناساگرهای آزمایشگاهی دایا What Are Phenol Red And Phenolphthalein In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. In more. What Are Phenol Red And Phenolphthalein.
From en.wikipedia.org
FilePhenolphthaleinatpH9.jpg Wikipedia What Are Phenol Red And Phenolphthalein In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. Adding extra hydrogen ions shifts. For example,. What Are Phenol Red And Phenolphthalein.
From www.lookfordiagnosis.com
Phenolphthalein What Are Phenol Red And Phenolphthalein Substances such as phenolphthalein, which can be used to determine the ph of a solution,. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. You titrate until the solution becomes colorless because that is as close to the. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph. What Are Phenol Red And Phenolphthalein.
From www.researchgate.net
How to measure PHENOLS in wastewater by titration method? any What Are Phenol Red And Phenolphthalein For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. In this case, the weak acid is colourless and its ion is bright pink.. What Are Phenol Red And Phenolphthalein.
From klaufzbrl.blob.core.windows.net
Phenolphthalein Indicator Range at Beatrice Simmons blog What Are Phenol Red And Phenolphthalein In this case, the weak acid is colourless and its ion is bright pink. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges. What Are Phenol Red And Phenolphthalein.
From www.vedantu.com
How will you convert phenol to phenolphthalein? What Are Phenol Red And Phenolphthalein Adding extra hydrogen ions shifts. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. If you use phenolphthalein, you expect a pink/red color to vanish. What Are Phenol Red And Phenolphthalein.
From www.vintessential.com.au
Phenolphthalein indicator Vintessential Wine Laboratories What Are Phenol Red And Phenolphthalein If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5. What Are Phenol Red And Phenolphthalein.
From www.macsenlab.com
Phenolphthalein Indicator 77098 Manufacturer & Supplier What Are Phenol Red And Phenolphthalein For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. You titrate until the solution becomes colorless because that is as close to the. Adding extra hydrogen ions shifts. If you use phenolphthalein, you expect a pink/red color to. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
Phthalein reaction test for phenols YouTube What Are Phenol Red And Phenolphthalein Adding extra hydrogen ions shifts. In this case, the weak acid is colourless and its ion is bright pink. Substances such as phenolphthalein, which can be used to determine the ph of a solution,. You titrate until the solution becomes colorless because that is as close to the. In more basic solutions where the hydronium ion concentration is less than. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
How to make a Phenolphthalein Indicator Solution (0.05wt) YouTube What Are Phenol Red And Phenolphthalein Substances such as phenolphthalein, which can be used to determine the ph of a solution,. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate ph ranges of about 8 to 10, 4.5 to 6, and 6 to 7.5 accordingly. In more basic solutions where the hydronium ion concentration is less than 5.0 ×. What Are Phenol Red And Phenolphthalein.
From www.vintessential.com.au
Phenolphthalein indicator, 0.5, 125mL Vintessential Wine Laboratories What Are Phenol Red And Phenolphthalein In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. You titrate until the solution becomes colorless because that is as close to the. Adding extra hydrogen ions shifts. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of. What Are Phenol Red And Phenolphthalein.
From worldlabsupplies.com
Phenolphthalein Paper World Lab Supplies What Are Phenol Red And Phenolphthalein If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. In this case, the weak acid is colourless and its ion is bright pink. Adding extra hydrogen ions shifts. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. What Are Phenol Red And Phenolphthalein.
From www.youtube.com
Phthalein dye test for Phenols (Phenolphthalein) IIT JEE Vineet What Are Phenol Red And Phenolphthalein Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. If you use phenolphthalein, you expect a pink/red color to vanish below a ph value of 8.0. For example, common indicators such as phenolphthalein, methyl red, and bromothymol blue are used to indicate. What Are Phenol Red And Phenolphthalein.